![]()
Chemical Foundation of Cell
Chemical Foundation of Cell – Elements in various combinations comprise all matter including living things Some of the most abundant element in living organism include carbon hydrogen nitrogen oxygen sulfur and phosphorus.
These are the nucleic acids proteins carbohydrate and lipids that are the fundamental components of living matter.
These are important building block of life and the uniqueness of the structures of the atoms that make up molecules allowing for the formation of cells, tissues organs systems and entire organisms make them as the basic chemical foundation of life.
Chemical Foundation of Cell
Water
- Water
The most abundant substance in the cell! - Where did it come from?
- several hypothesis:
- condensation from the primary atmosphere
- release of gases from the Earth interior
- extraterrestrial origin
accounts for about 70% of a cell’s weight
most intracellular reactions occur in an aqueous environment
Oceans → beginning of the life on Earth
life is dependent on the properties of water
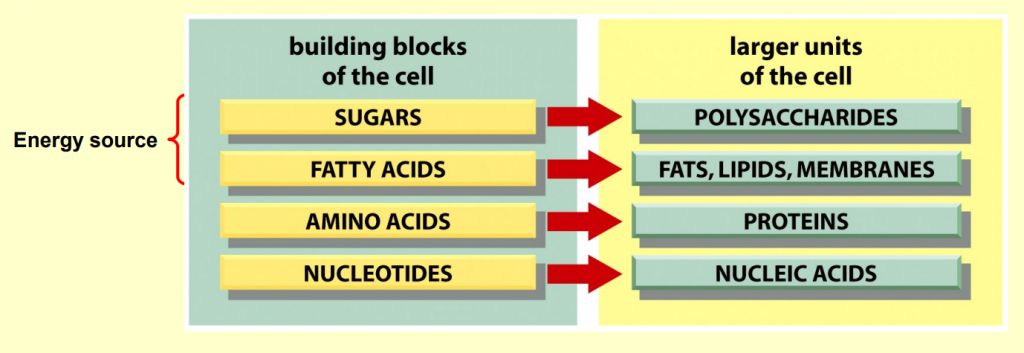
- Small organic molecules
carbon-based compounds – up to 30 C atoms
molecular weight 100 – 1000 kDa
usually found free in solution
many different fates :
monomer subunits for building macromolecules
source of energy for intracellular metabolic pathways
much less abundant that the organic macromolecules – only about 1/10 of the total mass of organic matter in the cell (Table 2-2).
around 1000 different kinds of these molecules in a typical cell
Four main types of small organic molecules:
sugars
fatty acids (lipids)
amino acids
nucleotides
Sugars
Monosaccharide
– the simplest sugars
– general formula (CH2O)n, n = 3, 4, 5, 6, 7, 8
– carbohydrates
– glucose C6H12O6
– the formula does not fully define the molecule – variety of ways in which C, H and
O atoms can be joined together by covalent bonds → structures with different shapes
– changing the orientation of specific –OH group glucose is converted to mannose or
galactose → isomers
– each of these sugars can exist in either two forms L- and D-forms → mirror images
of each other → optical isomers
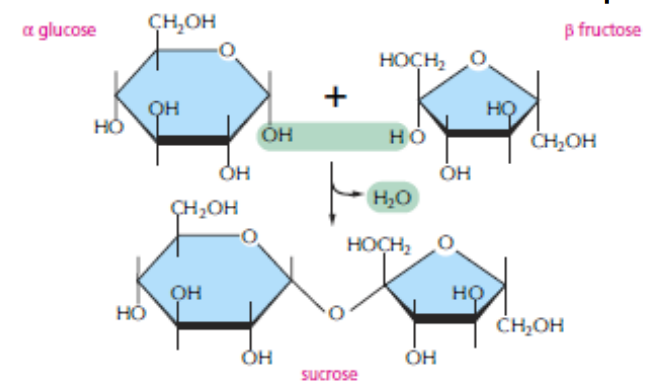
DISACCHARIDES
The carbon that carries the aldehyde or the ketone can react with any hydroxyl group on a second sugar molecule to form a disaccharide. The linkage is called a glycosidic bond. Three common disaccharides are maltose (glucose + glucose) lactose (galactose + glucose) sucrose (glucose + fructose)
OLIGOSACCHARIDES AND POLYSACCHARIDES
Large linear and branched molecules can be made from simple repeating sugar subunits. Short chains are called oligosaccharides, while long chains are called polysaccharides. Glycogen, for example, is a polysaccharide made entirely of glucose units joined together.
Importance:
energy sources
- glucose – key energy source for cells → in a series of reactions is broken down to
smaller molecules to release the energy - cells use simple polysaccharides composed only of glucose units → glycogen
(animal cells) and starch (plant cells)
mechanical support - cellulose – glucose polysaccharide (the most abundant organic chemical on Earth!)
- chitin – linear polymer of N-acetylglucosamine
molecular markers - glycoproteins and glycolipids of the cell membrane → selective recognition by other
cells - human blood groups
Fatty acids (lipids)
palmitic acid
Lipids
→ fatty acid and their derivatives
→ water insoluble; soluble in fats and organic solvents
two different structural parts:
- hydrophilic head – chemically active
- hydrophobic tail – differences between
hydrocarbon chains – not very active chemically - almost all fatty acids are covalently linked to
other molecules by their carboxylic acid group
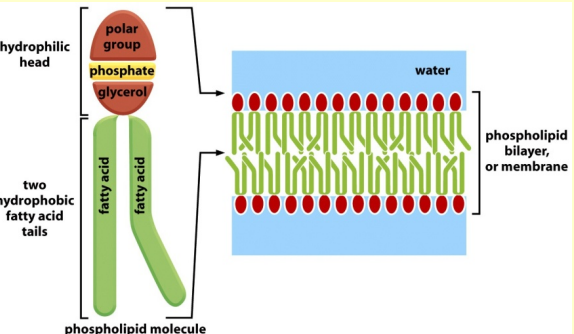
Phospholipids
Phospholipids are the major constituents of cell membranes.
In phospholipids two of the -OH groups in glycerol are linked to fatty acids, while the third -OH group is linked to phosphoric acid. The phosphate is further linked to one of a variety of small polar groups (alcohols).
Importance
- energy source
- stored in the cytoplasm of many cells in the form of droplets of triacylglycerol
molecules - animal fats (meat, butter and cream)
- plant oils (corn and olive oil)
construction of biological membranes - Cell and organelle membrane
- phospholipids
- glycolipids
- cholesterol
Amino acids
all have carboxylic acid group (COOH) and amino group (NH2) both
linked to a single C-atom (α-carbon)
chemical variety → side chain attached to the α-C
amino acids linked together – peptid bond
polypeptide or protein – two chemically distinct ends:
- NH2 (N-terminus)
- COOH (C-terminus)
20 types of aa in proteins of bacteria and eukaryotic cells
Why? Evolutionary mistery! – no obvious chemical reason!
All aa except Gly exist as optical isomers (D- and L-form)
Only L-forms are ever found in proteins!
(D-aa occur as part of bacterial cell wall and in some antibiotics)
Another evolutionary mistery!

Amino acids are subunits of proteins
Particulary abundant and versatile
Responsible for thousands of distinct functions in cells
- Enzymes – catalysts that direct many reaction in cells
Structural proteins
– tubulin – microtubules
– histones – chromosomes
Molecular motors
– movements of cells and cell structures
– myosin in muscles
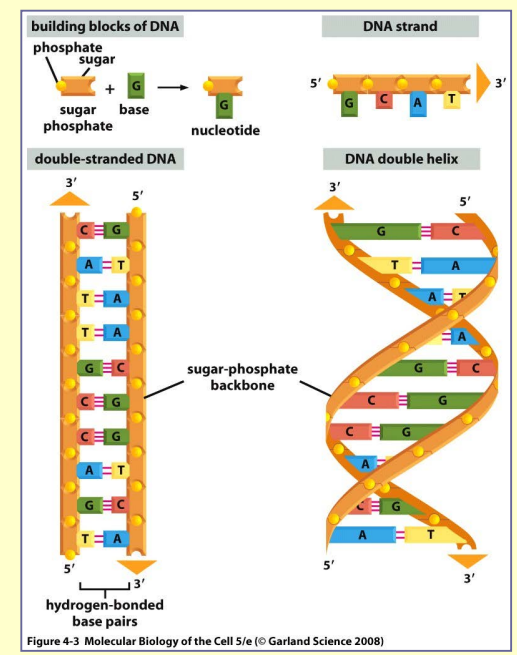
Nucleotides
- pentose sugar
- ribose → ribonucleotides
- deoxyribose → deoxyribonucleotides
- one or more phosphate groups
- bases
pyrimidines – they all derive from
six-membered pyrimidine ring - cytosine (C), thymine (T) and uracil (U)
purines – they have a second, five-membered
ring fused to the six-membered ring - adenine (A) and guanine (G)
the most important role → storage of biological information
building blocks of nucleic acid → nucleotides covalently
linked by the formation of phosphodiester bond
phosphodiester bond– linkage between phosphate group
attached to the sugar of one nucleotide and a hydroxyl group
on the sugar of the next nucleotide
two types of nucleic acids:
- RNA – ribose + A, G, C i U; mostly single-stranded
- DNA – deoxyribose + A, G, C i T; double stranded helix
Synthesis of each polymer involves the loss of water in a condensation reaction
Consumption of high-energy nucleoside triphospahte is required (activate each monomer before its addition)
Hydrolysis – the reverse reaction – breakdown of polymers (water addition)