![]()
Basic nutritional composition of plant tissue culture media
- Growth and morphogenesis of plant tissue in-vitro are largely regulated by composition of the culture media (Nutrient Media).
- The basic requirements of cultured plant tissue are similar to those of whole plants growing in soil but Nutritional requirements for optimal growth of tissues in-vitro may vary with the species. Even tissue from different parts of the plant may have different requirements for satisfactory growth.
- As such no single medium can be suggested as being entirely satisfactory for all types of plant tissues and organs.
- A medium containing only chemically defined compounds is referred to as a synthetic medium.
- In Plant tissue culture media millimoles per liter is used for expressing the concentration of nutrients and organic nutrients required in large amounts and micro mole or lit. for nutrients required in small amounts.
- The concentration of inorganic components of some plant tissue culture media is expressed in mg/l or ppm or mgl-1.
- According to the recommendation of the international association of plant physiology mmol-1 should be used for expressing the concentration of macronutrients and μmol-1 for micronutrient, hormones vitamins etc.
- Some tissues grow on simple media containing only inorganic salts and utilizable carbon sources but for most others it is essential to supplement the medium with vitamins, amino acid and growth substances.
- Media compositions are therefore formulated considering specific requirement of a particular type of culture eg. shot tip culture, anther culture, root or shoot initiation and multiplication etc.
- Various types of media have been devised for specific organs and tissue.
Example of media used are –
(i) White’s medium: Used for root culture and to induced organogenesis and regeneration.
(ii) M.S. medium: Basal media
(iii) L.S.: (Linsmaier and Skoog Medium).
(iv) B5 medium: For cell suspension or callus culture.
(v) N6 medium: For anther culture.
- The minerals present in the Plant Tissue Culture media can be used by plant cells as building blocks for the synthesis of organic molecules.
- The concentrations of the dissolved salts play an important role in the osmotic regulation and in maintaining the electrochemical potential.
- Nitrogen, sulfur and phosphorus are components of proteins and nucleic acid. Mg and micronutrient are essential part for catalysis of various metabolic reactions.
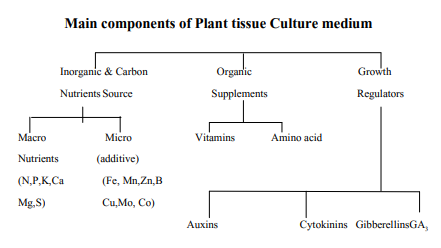
- The Principal components of most plant tissue culture media are inorganic nutrient carbon source, organic supplements, growth regulators and a solidifying agent.
1. Inorganic nutrients –
A variety of mineral salts supply the needed macro and micronutrients required by the plants in vitro. Element required in concentrations greater than 0.5 m mol-l ae called macronutrients and those less than 0.05 m mol-l are micronutrients.
- Macronutrient : Sic major elements, Nitrogen, Phosphorous, Potassium, Calcium, Magnesium and sulphur are essential for plant cell and tissue growth. Culture media should contain at least 25m mol-l is contributed by both nitrates and ammonium. Other major element like Ca, P, S, and Mg in the range of 1-3m mol-1 are adequate.
- Ca – Important for strength of the Cell-wall and resistance against fungal infection and regulation of cell potential.
- P – Used as H2PO4 form.
- K – In highest concentration in medium used as ionic barrier, osmotic regulation.
- Mg – Plays important role in photosynthesis.
- N – Used as a source of protein and amino acid. It helps in osmoregulation, maintaining cation-anion balance.
- S – Absorbs by plants in the form of sulphates use in amino acids and Proteins.
- Micronutrient– Inorganic elements required in small quantity but essential for plant cells and tissue growth constitute microelements. These are Iron Manganese (Mn) Zinc, Boron, Copper and Molybdenum. Chelated forms of iron and Zink are commonly used in preparing culture media. EDTA -iron chelate is used in place of iron citrate because iron citrate is difficult to dissolve and precipitates in the medium. Certain Media are enriched with cobalt (Co) Iodine and Sodium.
- B – Use in lignifications of cell wall and differentiation of xylem.
- Cl – Absorb in Cl– form.
- Fe – Use in chelated form with EDTA.
- Cu – As co-factor in enzymes.
- Co – Important in N2 fixation.
- Mn and Mo – They bind to several metaloproteins.
2. Carbon and Energy Source :
- The most important Carbon source is sucrose.
- Glucose and fructose are also used as a source of carbon.
- Glucose supports equally good growth while fructose is less efficient.
- During autoclaving of medium sucrose gets converted into glucose and fructose.
- The other carbohydrates used are lactose and galactose, maltose, cellobiose etc, yield inferior result.
- Plant cell and tissue in the culture medium lack autotrophic ability and therefore need external carbon for energy.
- The additional of an external carbon source like sucrose to the medium enhances proliferation of cells and regeneration of green shoots.
3. Organic Supplement :
Vitamins –
- Plant synthesis vitamins endogenously.
- But when plant cells and tissues are grown in vitro some essential vitamins are synthesised in suboptimal quantities.
- The medium should be supplemented with vitamins like thiamine (B1), nicotinic acid (B3), pyridoxine (B6), Calcium penthothenate (B5) and myoinositol.
- Thiamine is the basic vitamin required by all cells and tissues.
- Other vitamins such as biotin, folic acid, ascorbic acid, pantothenic acid, vitamin E, riboflavin etc, are also used.
- These vitamins are added in the range of 0.1 to 10.0 mgl-1.
Amino acids:
- Cultured tissues are capable of synthesising the amino acids necessary for various metabolic processes but their addition to media is necessary for stimulating cell growth in protoplast cultures and for establishing cell lines.
- Amino acids like L-glutamine, L asparagine, L glycine, L-arginine and L-cysteine are commonly used in culture media.
- Tyrosine is only used when agar is added to the medium.
- Amino acids promote cell growth in cultures when their mixtures ara used rather than a single amino acid.
Other organic supplements:
- Culture media are often supplemented with a variety of organic extracts like milk protein (Casein hydrolysate), coconut milk, yeast and malt extract, ground banana, tomato juice, orange juice, etc.
- The use of natural extract is generally avoided because the quality and quantity of growth promoting constituents vary with age of the tissue from which they are extracted.
- Also it is desirable to replace these organic supplements with a defined organic substance for eg. a single amino acid, L-asparagine, could effectively replace yeast extract and tomato juice in the medium for callus culture of maize endosperm.
Activated charcoal –
- The addition of activated charcoal is reported to stimulate growth and differentiation in orchids, carrots, etc.
- On the other hand, it proved inhibitory on tobacco, soybean etc.
- Inhibition of growth may be due to adsorption of phytohormone on activated charcoal.
- whereas stimulation may be due to absorption of compounds inhibitory for plant or tissue growth.
- It also helps to reduce toxicity by removing toxic compounds for eg. phenols.
Antibiotics-
- Addition of antibiotics to culture media is generally avoided because their presence in the medium retards the cell or tissue growth.
- But in some plant cells which have a systemic infection of microorganisms, it is essential to use antibiotics in media.
- Streptomycin or kanamycin at low concentration effectively controls infection.
- For gene cloning and transformation experiments using E. coli and agrobacterium, rifampicin, tetracycline, gentamycin, sulphate, augmentin, chloramphenicol etc, are used at varying concentrations depending on the vector and selection of the transformant.
4. Growth regulators;
- Four classes of growth regulators namely auxins, cytokinins, gibberellins and abscisic acid are important in tissue culture.
- The growth, differentiation and organogenesis in vitro is possible only in the presence of these hormones.
Auxins –
- Various auxins like Indole Acetic Acid ((IAA), Naphthalene acetic acid (NAA), Indole butyric acid (IBA), 2,4-dichlorophenoxyacetic acid (2,4-D) are used in media.
- IAA occurs naturally in plant tissues, Auxins have the property of inducing cell division, involved in elongation of stem, internodes, tropism, apical dominance, abscission and rooting.
Cytokinin
- Cytokinins are adenine derivatives and their main physiological role is cell division, modification of apical dominance and shoot differentiation and multiplication in culture.
- The most commonly used cytokinins are 6- benzylaminopurine (BAP), 6-𝛾-𝛾 -dimethylaminopurine (2-ip), N-(2-Furfuryl Amino) 1- H-purine -6 amine (kinetin) and zeatin.
- Zeatin and 2-ip are naturally occurring cytokinins while kinetin and 6 – benzyl adenine (BA) are synthetic.
- The ration of auxins and cytokinins are important with respect to morphogenesis in culture.
- For callus initiation, embryogenesis and root initiation the ratio of auxins and cytokinins is high, while the reverse i.s cytokinins to auxin leads to axillary and shoot proliferation.
Gibberellins and abscisic acid –
- These growth regulators are used less often in tissue culture.
- In some plant species, these hormones enhance and in others inhibit growth.
- GA3 is the most common gibberellin which promotes growth of cell cultures at low density, enhances callus growth and induces dwarf or stunted plants to elongate.
- Abscissic acid (ABA) in culture medium either stimulates or inhibits the callus growth depending on the species.
5. Solidifying agents;
- Gelling agents like agar, agarose etc are commonly used in tissue culture.
- Gels provide a support to tissue growing in static conditions.
- Agar obtained from seaweeds is a polysaccharide, inert substance which do not react with the constituents of media.
- Secondly it is not digested by plant enzymes and remains stable at all incubation temperatures.
- Normally 0.5 to 1% agar is used in the medium to form a gel at the required pH.
- Gelatin, methacel, alginate, phytagel, gelrite etc are also commonly used as semi solidifying agent.
- Perforated cellophane, filter paper bridges, polyester fleece are alternative methods of support used in media.
6. pH –
- Plant cells and tissues require optimum pH for growth and development in cultures.
- The pH affects the uptake of ions and normally ranges between 5.0 to 6.0 before sterilization.
- Higher pH gives a hard medium whereas a low pH results in unsatisfactory solidification of the agar.