![]()
Artificial chromosomes
There are four types of artificial chromosomes.
They are:
(1) Yeast Artificial Chromosomes
(2) Bacterial Artificial Chromosomes
(3) Mammalian Artificial Chromosomes and
(4) Artificial Human Chromosomes.
Yeast Artificial Chromosomes (YAC)
- It is develop by D.T. Burke and G.F. Carle in the laboratory of M.V. Olson.
- Yeast artificial chromosomes (YACs) are genetically engineered chromosomes derived from the DNA of the yeast, Saccharomyces cerevisiae, which is then ligated into a bacterial plasmid.
- By inserting large fragments of DNA, from 100–1000 kb, the inserted sequences can be cloned and physically mapped using a process called chromosome walking.
- The development of YAC’s were based on the logic that an eukaryotic linear chromosomes needs for its replication and stability, not only replication origins, but also the centromere and the telomere.
- The centromere sequence would attach to the mitotic spindle during cell division and help in efficient segregation of the chromosomes into the daughter cells.
- The telomere would preserve the integrity of the ends of the linear chromosomes.
- Once these elements were provided, the vector could be replicate stably like a chromosome and could accommodate chromosomes sized inserts.
- Indeed, standard YACs can accommodate around 600 kb DNA inserts, while special type of YACs can accommodate up to 1400 kb DNA inserts.
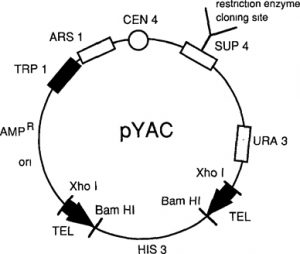
- Though an YAC vector is meant to be propagated like a chromosome in yeast, it is a circular double stranded DNA that contains a replication origin (colE 1) compatible with E. coli in addition to yeast replication origin or an yeast ARS element.
- The col El replication origin is useful to a yeast replication origin or an yeast ARS elements. The colEl replication origin is useful for amplification of the vector in E. coli.
- Next to the yeast replication origin is located the centomere of yeast chromosome 4 (Cen 4).
- The two telomere sequences are from the protozoan Tetrahymenal, which have been found to be functional in yeast.
- A staffer element containing the His 3 gene of yeast is present between the two telomere sequences through two BamHI restriction endonuclease sites.
- Three selectable yeast marker genes, Trp 1, Ura 3 and Sup 4 are also present.
- The marker genes Trpl and Ura 3 are on the two sides of the unique restriction endonuclease site SnBl, that produces blunt ends.
- The SnaB 1 site is used as the cloning site and is located within the Sup4 gene. Sup 4 gene product is a tRNA that suppresses a mutation in the Ade gene of yeast resulting in a change in colour of colonies from red to white in the presence of limiting amounts of adenine in the medium.
- The YAC vector is digested with BamHI and SnaB I throwing the staffer element out, disrupting the Sup4 gene and yielding two vector fragments termed as left arm and right arm.
- Each of the arms but at its one end a telomere sequence followed by a BamHI overhang, but a blunt flush cut at the other end.
- The left arm also has the Cen 4 sequence, the ARS1, the ColEl origin, the amp and Trp 1 markers. The right arm contains the Ura 3 marker.
- These two arms are blunt end ligated with the long chromosomal DNA fragment from any source, thus creating the artificial linear chromosome among other ligation products.
- Ligation products are introduced into yeast cells that have mutation in the Trpl, Ura3 and Ade loci by lipofection or by fusion with yeast speroplasts.
- The yeast cells are allowed to regenerate the cell walls and plated on medium lacking trytophan and uracil and containing limiting amount of adenine.
- Only the yeast cells transformed with artificial chromosomes comprising both the left arm and the right arm would grow.
- The recombinant artificial chromosomes containing the insert would develop into white colonies.
- The red colonies would represent cells having the linear vector, but no insert.
- Cells having other ligation products like two left arms or two right arms would not grow.
Construction
- A YAC is built using an initial circular DNA plasmid, which is typically cut into a linear DNA molecule using restriction enzymes; DNA ligase is then used to ligate a DNA sequence or gene of interest into the linearized DNA, forming a single large, circular piece of DNA.
- The basic generation of linear yeast artificial chromosomes can be broken down into 6 main steps:
1. Ligation of selectable marker into plasmid vector:
- This allows for the differential selection of colonies with, or without the marker gene An antibiotic resistance gene allows the YAC vector to be amplified and selected for in E. coli by rescuing the ability of mutant E. coli to synthesize leucine in the presence of the necessary components within the growth medium. TRP1 and URA3 genes are other selectable markers.
- The YAC vector cloning site for foreign DNA is located within the SUP4 gene. This gene compensates for a mutation in the yeast host cell that causes the accumulation of red pigment.
- The host cells are normally red, and those transformed with YAC only, will form colorless colonies. Cloning of a foreign DNA fragment into the YAC causes insertional inactivation of the gene, restoring the red color. Therefore, the colonies that contain the foreign DNA fragment are red.
2. Ligation of necessary centromeric sequences for mitotic stability
3. Ligation of Autonomously Replicating Sequences (ARS)
- providing an origin of replication to undergo mitotic replication.
- This allows the plasmid to replicate extra chromosomally, but renders the plasmid highly mitotically unstable, and easily lost without the centromeric sequences.
4. Ligation of artificial telomeric sequences to convert circular plasmid into a linear piece of DNA
5. Insertion of DNA sequence to be amplified (up to 1000 kb)
6. Transformation yeast colony
Bacterial Artificial Chromosomes (BAC)
- Bacterial artificial chromosomes (BAC) were developed by Mel Simmons and coworkers in the early 1990s It is based on the fertility factor (F factor) of Escherichia coli.
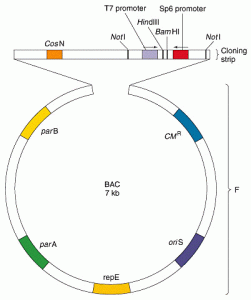
- The F plasmid, a ~ 100 kb circular double stranded DNA, is present is an E. coli cell in only 1-2 copies.
- The synthetic BAC vectors, which are only ~7.5 kb double stranded DNA circles contain the replication origin oriS and the gene repE of the F plasmid that are responsible for initiation and proper orientation of replication of the BAC vector.
- The parA and parB genes of the F plasmid ensure efficient segregation of the F factor into the daughter E. coli cells after its replication are also incorporated in the BAC vector.
- The BAC vectors also contain multiple cloning sites (mcs), a selectable marker in the form of antibiotic resistance and colour based identification (lac Z complementation system) of recombinants carrying inserts.
- The naturally occurring F2 factors consist of up to 25% of the E. coli genome integrated into the basic F factor and are very stable.
- This characteristic of the F factor contributes to the ability BACs to accommodate very large amount of external DNA to the extent of 300kb.
- The recombinant BACs have been found to exhibit a lower level of rearrangement and chimerism of the cloned DNA sequence than exhibited by YACs.
- The cloning of DNA in BACs is done as is done in a plasmid, by linearising the vector with a restriction endonuclease, treating with phosphatase and then ligating with the DNA fragments to be cloned. E. coli has to be transformed by electroporation because of the large size of the recombinant BAC.
Mammalian Artificial Chromosomes (MACs):
- The YACs, the BAC and the PACs (plasmid artificial chromosomes) have found regular use for cloning large genomic DNA fragments in various genome sequencing projects. Another potential application of artificial chromosomes is in gene therapy of human.
- For gene therapy, we need to have gene in human DNA fragments including their promoters and all the control elements.
- This would have to be introduced into the target cells efficiently and would have to be stably maintained inside the nucleus, generation after generation through unlimited number of divisions.
- The DNA would have to be expressed properly without interfering with the function of other resident.
- Such large artificial human chromosome (aptly termed mini-chromosomes), if 1-10 mb size have been claimed to be stable for more than 100 cell generations. Satellite DNA based mini-chromosomes of 20-30 mb have also been reported.
Human Artificial Chromosomes:
- A human artificial chromosome (HAC) is a microchromosome that can act as a new chromosome in a population of human cells.
- That is, instead of 46 chromosomes, the cell could have 47 with the 47th being very small, roughly 6–10 megabases (Mb) in size instead of 50–250 Mb for natural chromosomes, and able to carry new genes introduced by human researchers.
- Ideally, researchers could integrate different genes that perform a variety of functions, including disease defense.
- Alternative methods of creating transgenes, such as utilizing yeast artificial chromosomes and bacterial artificial chromosomes, lead to unpredictable problems.
- The genetic material introduced by these vectors not only leads to different expression levels, but the inserts also disrupt the original genome.
- HACs differ in this regard, as they are entirely separate chromosomes.
- This separation from existing genetic material assumes that no insertional mutants would arise.
- This stability and accuracy makes HACs preferable to other methods such as viral vectors, YACs and BACs.
- HACs allow for delivery of more DNA (including promoters and copy-number variation) than is possible with viral vectors.
- Yeast artificial chromosomes and bacterial artificial chromosomes were created before human artificial chromosomes, which first appeared in 1997.
- HACs are useful in expression studies as gene transfer vectors, as a tool for elucidating human chromosome function, and as a method for actively annotating the human genome.
- HACs were first constructed de novo in 1997 by adding alpha-satellite DNA to telomeric and genomic DNA in human cells.
- This resulted in an entirely new microchromosome that contained DNA of interest, as well as elements allowing it to be structurally and mitotically stable, such as telomeric and centromeric sequences. Due to the difficulty of “de novo” HAC formation, this method has been largely abandoned.
Construction methods
- In this the research team created artificial chromosomes from normal human material.
- The researchers first synthesized arrays of alpha satellite DNA, and then introduced the resulting centromeric material into human cells in conjunction with telomeres and genomic DNA.
- Inside the cells, the independent elements assembled to form miniature chromosomes, or synthetic micro-chromosomes, that were structurally similar to human chromosomes, but contained less genetic material.
- Analysis of the newly introduced artificial chromosomes demonstrated normal centromeric activity, genetic stability, and continued gene expression through repeated rounds of the cell cycle.
Applications
- A 2009 study has shown additional benefits of HACs, namely their ability to stably contain extremely large genomic fragments. Researchers incorporated the 2.4 Mb dystrophin gene, in which a mutation is a key causal element of Duchenne muscular dystrophy.
- The resulting HAC was mitotically stable, and correctly expressed dystrophin in chimeric mice. Previous attempts at correctly expressing dystrophin have failed. Due to its large size, it has never before been successfully integrated into a vector.
- In 2010, a refined human artificial chromosome called 21HAC was reported. 21HAC is based on a stripped copy of human chromosome 21, producing a chromosome 5 Mb in length.
- Truncation of chromosome 21 resulted in a human artificial chromosome that is mitotically stable. 21HAC was also able to be transferred into cells from a variety of species (mice, chickens, humans). Using 21HAC, researchers were able to insert a herpes simplex virus- thymidine kinase coding gene into tumor cells.
- This “suicide gene” is required to activate many antiviral medications.
- These targeted tumor cells were successfully, and selectively, terminated by the antiviral drug ganciclovir in a population including healthy cells.
- This research opens a variety of opportunities for using HACs in gene therapy.
- In 2011, researchers formed a human artificial chromosome by truncating chromosome 14. Genetic material was then introduced as mentioned above, using the Cre-Lox recombination system.
- This particular study focused on changes in expression levels by leaving portions of the existing genomic DNA.
- By leaving existing telomeric and sub-telomeric sequences, they were able to amplify expression levels of genes coding for erythropoietin production over 1000-fold. This work also has large gene therapy implications, as erythropoietin controls red blood cell formation.
- HACs have been used to create transgenic animals for use as animal models of human disease and for production of therapeutic products