![]()
Protoplast culture
Protoplast culture techniques :
- The culture requirements and the culture methods are the same for both protoplasts and single cells.
- The man difference is the requirement of suitable osmoticum for protoplasts until they regenerate a strong cell wall.
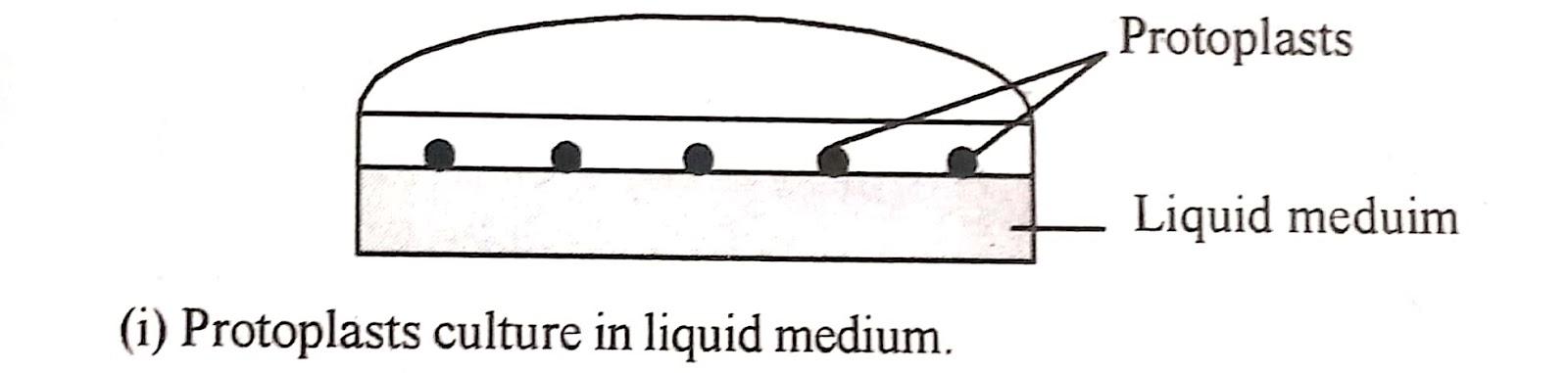
Isolated protoplasts are either cultured in liquid or semisolid agar or agarose media plates. Sometimes, the protoplast is first grown in liquid media and then transferred into the agar media plates.
Culture Medium
The nutritional requirement of protoplast is almost similar to that of cultured plant cells Mostly. the salts of MS (Murashige and Skoog) and B5 (Gamborg et al 1968) media and their modifications have been used. Ammonium salts have been found detrimental to protoplasts survival of many species, and media have been devised that either have a reduced concentration of ammonia or lack it Concentration of zinc is reduced while the concentration of calcium is increased as it enhances the membrane stability
Osmolarity is maintained by the addition of sorbitol, mannitol. glucose or sucrose. Mannitol is widely used as osmoticum as it’s not uscd by the dividing cells Thus maintains the osmolarity of the medium
Glucose is the preferred carbon source as sucrose do not satisfy protoplast culture One or two amino acids are added at low concentration. Growth regulators are required essentially. generally high concentration of auxins (NAA, 2, 4-D) along with lower concentration of cytokinins (BAP, Zeatin) is used
Environmental conditions : High light intensity inhibits growth of protoplast hence initially protoplast is grown in dim light for a few days and then transferred to light of about 2000-5000 lux. Better results are obtained when cultured in darkness.
Plating density : Like cell cultures. the initial plating density of protoplasts has profound effect on plating efficiency Protoplasts are cultured at a density of 1x 10⁴ to l x 10⁵ protoplasts ml⁻¹ of the medium.
At high density the cell colonies arising from individual protoplasts tend to grow into each other resulting into chimera tissue if the protoplast population is genetically heterogeneous. Cloning of individual cells, which is desirable in somatic hybridization and mutagenic studies can be achieved if protoplasts or cells derived from them cani be cultured at a low density.
Osmoticum or Osmotic stabilizer –
Osmoticum broadly refers to the reagents / chemicals that are added to increase the Osmotic pressure of a liquid.
Isolation and culture of protoplast require protection until they develop strong cell wall.
in fact if the freshly isolated protoplast are directly added to the normal culture medium they will brust . so the edition of an cosmeticum is essential for both isolation and culture media of protoplast to prevent their rupture.
The osmotica are of two types –
1- Non ionic – Osmotic
The non ionic substances most commonly used are soluble carbohydrates such as mannitol, Sorbitol, glucose, fructose galactose and sucrose. mannitol is mostly use as osmoticum.
2- Ionic osmotica-
potassium chloride calcium chloride and magnesium phosphide are the ionic substances in use to maintain osmotic pressure
culture techniques
i) Liquid method :
This method is preferred in earlier stages of culture as it provides
(a) easier dilution and transfer
(b) The osmotic pressure of liquid media can be effectively reduced after a few days of culture
c) The cell density can be reduced or cells of special interest can be isolated easily
In liquid medium. the protoplast suspension is plated as a thin layer in petri plates incubated as static culture in flasks or distributed in 50-100 𝜇l drops in petri plates and stored in a humidity chamber
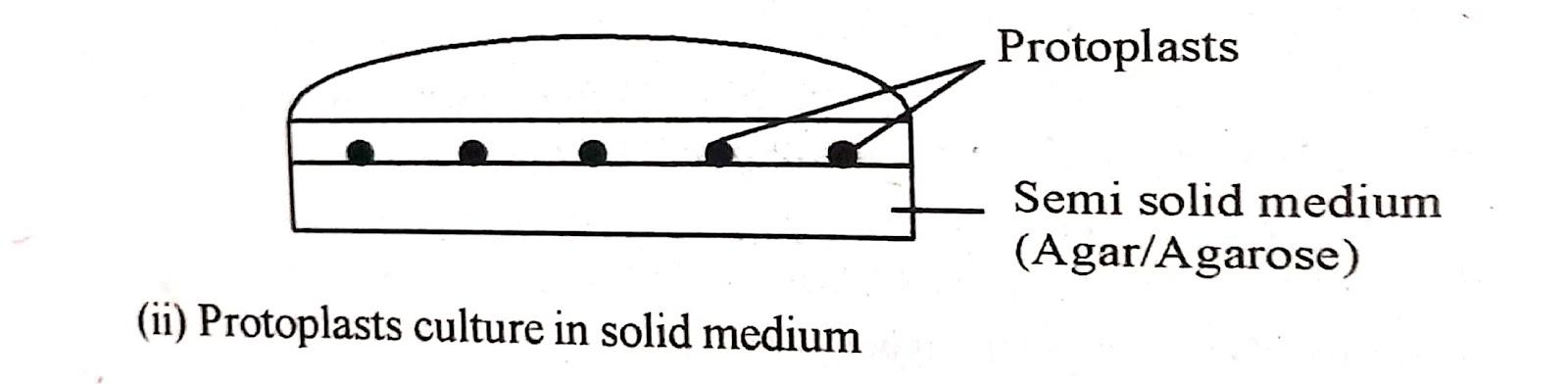
(ii) Embedded in Agar/Agarose :
Agarose is a preferred choice in place of agar and this has improved the culture response. This method of agar culture keeps protoplast in fixed position thus prevents It from forming clumps.
Immobilized protoplasts give rise to clones which can then be transferred to other media. In practice the protoplasts suspended in molten (40𝜊C) agarose medium (1-2% w/v agarose) are dispensed into small (3.5-5- cm diameter) plates and allowed to solidify The agarose layer is then cut into 4 equal sized blocks and transferred to larger dishes (9 cm diameter) containing liquid medium of otherwise the same composition.
Alternatively. protoplasts in molten agarose medium are dispensed as droplets 50-100𝜇l) on the bottom of the petri plates and after solidification, the droplets are submerged in the same liquid medium.
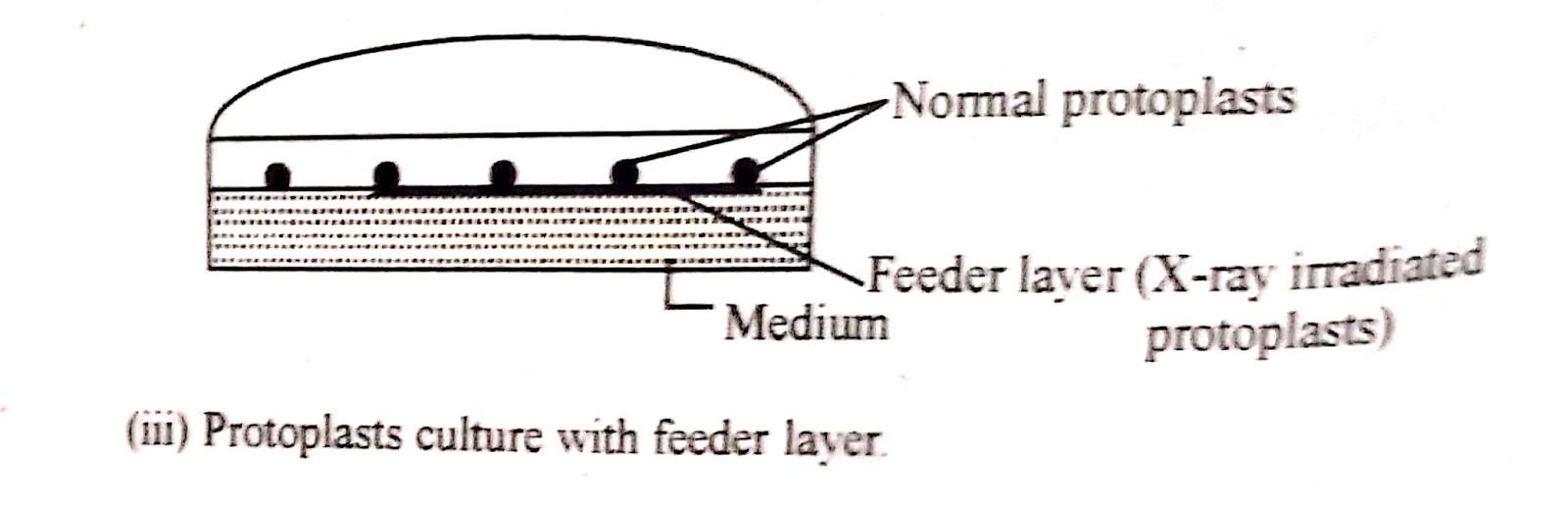
iii) Feeder layer :
In order to culture protoplasts at low density, a feeder layer technique is adopted A feeder layer of X-ray irradiated, non-dividing but metabolically active protoplasts, after washing three times to remove toxic substances due to Irradiation are plated on soft agar medium at a density of 2.4x 10⁴ml⁻¹
Non-irradiated protoplasts of low density (10- 100 protoplasts per ml) are plated over this feeder layer. The protoplasts of the same species or different species can be used as a feeder layer.
iv) Co-culturing :
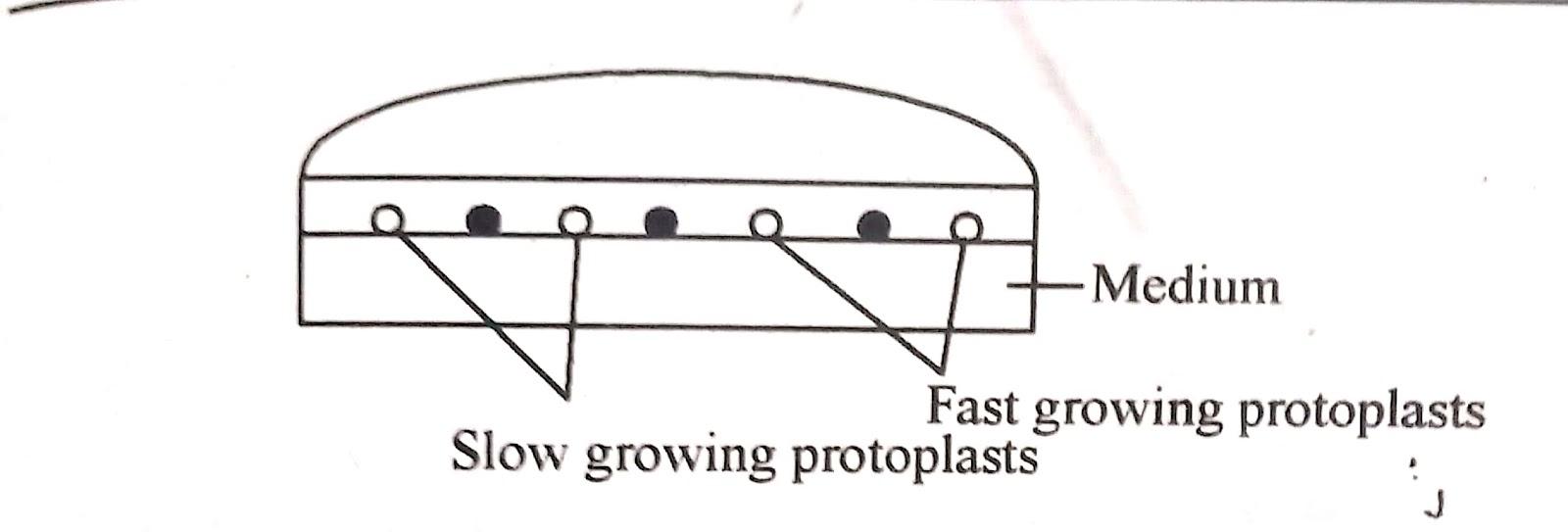
This method involves co-culture of protoplasts from two different species to promote their growth or that of the hybrid cells. Metabolically active and dividing protoplasts of two types. slow and fast growing, are cultured together
The fast growing protoplast provide other species with diffusible chemicals and growth factors which helps in cell wall formation and cell division.
The co-culture method is generally used where call arising from two types of protoplasts can be morphologically distinguished For eg. protoplasts isolated from albino plants and green plants are easily distinguishable based on colour where albino protoplast will develop non green colonies.
v) Drop cultures :
Small droplets of 40 to 100 ul of protoplasts suspension are placed on the Inner side of the lid of a petri dish When the lid is covered on the bottom, the culture drops are changed towards the bottom dish To the dish, fresh medium can be added in small drops when required.
vi) Microchamber cultures :
Microchamber cultures are similar to hanging drop cultures and are adopted for individual protoplast culture. A drop of protoplast suspension is placed on sterile cover glass and inverted on the slide with microchamber. Microchamber culture offers an optically better view since the depth of the chamber is kept at a minimum.
vii) Multiple Drop Array technique :
The MDA screening technique used hanging droplets of 40𝜇l as the experimental unit Each droplet represents one combination of factors to be tested The droplets are arranged in a regular array of 7 x 7 drops on the lid of a 9 cm petndish To test seven different auxins (factors) in combination with four different cytokinins in the I medum, each of these factors is used in seven different concentrations The whole experiment therefore includes 4×7 pet dishes. Since cach petri dish contains 49 droplets, this results is a total 4x7x49= 1372 two factor combinations.
viii) Micro Drop Culture :
Microdrop culture has been successfully used to culture hybrid cells of Nicotiana glauca, Glycine max. Arabidopsis thaliana and Brassica campestris
The technique requires specially designed Cuprak dishes which have a smaller outer chamber and a large inner chamber. The inner chamber carries numerous numbered wells each with a capacity of 0.25-25𝜇l droplet of nutrient medium. individual protoplasts or heterokaryons per 025-25 ul droplet of nutrient medium transferred by Drummond pipette to each well of the inner chamber of the cuprik dsh. The outer chamber is filled with sterile water to maintain humidity inside the dish After covering it with a lid. the dish is sealed with parafilm and maintained at optimal light and temperature conditions
The size of the droplet is critical for division of a single protoplast or heterokaryon as it gives a ratio of cell/volume of culture medium equal to a cell density 2.4.X10³ ml⁻¹ An increase In the size of the droplet would decrease the effective plating density.