![]()
Protoplast Fusion and Somatic Hybridization
Protoplast fusion can be accompanied by three processes.
- Spontaneous fusion
- Mechanical fusion,
- Induced fusion.
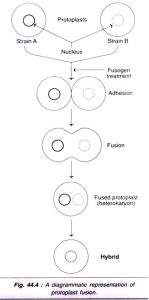
1. Spontaneous fusion :
During the enzymatic degradation of cell wall, some of the adjacent protoplasts may fuse together to form homokaryons (homokaryocytes).
These fused cells contain high number of nuclei cells (contain 2-40 nuclei )
This is mainly because of expansion and subsequent coalescence of the plasmodesmatal connections between the cells.
More frequent homokaryon formation has been observed in protoplasts isolated from dividing cultured cells.
This spontaneous protoplast cannot regenerate into whole plant.
2. Mechanical fusion :
The protoplasts fused mechanically by pushing together two protoplasts.
This fusion does not depend upon the fusion inducing agents. However in this procedure protoplasts are likely to get injured.
Protoplasts from meiocytes (Lilium and Trilliam) released enzyme in solutions really fuse by gentle tapping in a depression slide and some of the di and trinucleate cells reportedly complete the second division forming tetrad configuration in culture media
3.Induced fusion :
Freshly isolated protoplasts can be induced to undergo fusion, There are several fusion -inducing agents known as fusogens (fusion inducing agents) eg. NaNO3, lysozyme, high pH/Ca² polyethylene glycol (PEG), Polyvinyl alcohol, concavalin A, polyvinyl alcohol, dextran and dextran sulphate, fatty acids and esters.
Some of the following treatments are as follows which are used in protoplast fusion.
(A) NaNO₃, treatment :
The isolated protoplast are exposed to a mixture of 5.5% NaNO₃ in 10 % sucrose solution. Incubation is carried out for 5 minute at 35⁰C. Followed by centrifugation 200 xg for 5 min.
The protoplast pellets is kept in a water bath at 30ºC for about 30 minute, during which period protoplast fusion occurs NaNO₃ treatment result in a low frequency of heterokaryon formation, particularly when mesophyll protoplasts are fused.
(B) High pH/Ca’+ treatment :
Physical contact of two protoplasts is essential for their fusion. However protoplasts do not fuse easily due to two main reasons.
- i) They have a net negative charge on their membrane surfaces and force of repulsion works between protoplasts.
- i) It is difficult to remove water from hydrophilic surfaces of protoplasts which also create a repulsive force between two protoplasts.
It was observed that positively charged ions reduce the net negative charges of membranes reducing the repulsive force considerably.
used this method for fusing two different lines of tobacco protoplasts and is now commonly used.
Isolated protoplasts are incubated in a solution of 0.4 M mannitol containing 0.05 M CaCl₂, with pH at 10.5 (0.05 M glycine NaOH buffer) and temperature 37℃. Aggregation of protoplasts generally takes place at once and fusion occurs within 10 minutes. In this method 20-50% of the protoplast are involved in fusion. Intraspecific and interspecific somatic hybrids have been produced using this procedure.
(C) PEG treatment (Polyethylene glycol) :
PEG has been used as a fusogen in a number of plant species because of the reproducible high frequency of heterokaryon formation.
The isolated protoplast in culture medium (1ml) are mixed with equal volume (1ml) of 28-56% PEG (Mol.wt. 1500-6000) in a tube.
PEG enhance fusion of protoplast in several species. This tube is shaken and then allowed to settle.
The settled protoplast are washed several times with culture medium.
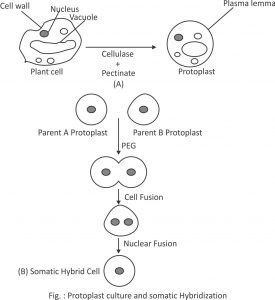
PEG induced fusion is non-specific. In addition to fusing soyabean maize and soyabean barley, PEG brings about effective fusion between animal cells, animal cells with yeast protoplast and animal cells with higher plant protoplasts.
Thus it have several advantages
- It result in a reproducible high frequency of heterokaryon formation
- Low toxicity to cells.
- Reduced formation of binucleate heterokaryons.
- PEG- induced fusion is non specific and therefore can be used for a wide range of plants.
(D) Electrofusion :
- Studies in the past few years have shown that electric fields can be used for protoplast fusion.
- This procedure called electrofusion
- It is a simpler, and quicker and more efficient method.
- In this Cells after electrofusion do not show cytotoxic responses as found in protoplasts or heterokaryons subjected to Chemical treatment.
- This protocol involves a two step process. First, the protoplasts are introduced into a small fusion chamber containing parallel wires (platinum wires) which serve as electrodes.
- Second. a low voltage and rapidly oscillating AC field is applied ( 100 v/cm, 0.6 MHz). which causes protoplasts to become aligned into chains of cells (pearl chains) between the electrodes.
- This creates complete cell to cell contact within a few minutes.
- Once alignment is complete, the fusion is induced by application of a brief spell of high voltage DC pulse (0.125-1 KV/cm)
- A high voltage DC pulse induces a reversible breakdown of the plasma membrane at the site of cell contact leading to fusion and consequent membrane reorganisation .
- The entire process starting from the introduction of protoplasts inside the fusion chamber and then transfer to culture media can be completed in 5 minutes or less.
- Heterokaryons produced by electrical fusion divide in the culture medium and have demonstrated the capability of regenerating somatic hybrid plants in some cases like
Niconiana tabaccum +N. plumbaun/olia,
N glauca and N. langsdorfii and
Solanum tuberosum and S. phureja.