![]()
Banding Patterns
Introduction
- Karyotype analysis is a technique where chromosomes are visualized under a microscope.
- Cells are collected from an individual, induced to divide, and then arrested at metaphase (a stage of cell division when the chromosome are condensed and therefore visible).
- The chromsomes are stained with certain dyes that show a pattern of light and dark bands, which is called as the banding pattern.
- These bands reflect regional differences in the amounts of A and T versus G and C.
- The banding pattern for each chromosome is specific and consistent allowing identification of each of the chromosomes.
- Preparation of chromosomes for karyotype analysis can be performed in a number of ways and each will yield differing pieces of information.
- The chromosomes may be stained with aceto- orcein,
- Feulgen or a basophilic dye such as toluidine blue or methylene blue if only the general morphology is desired.
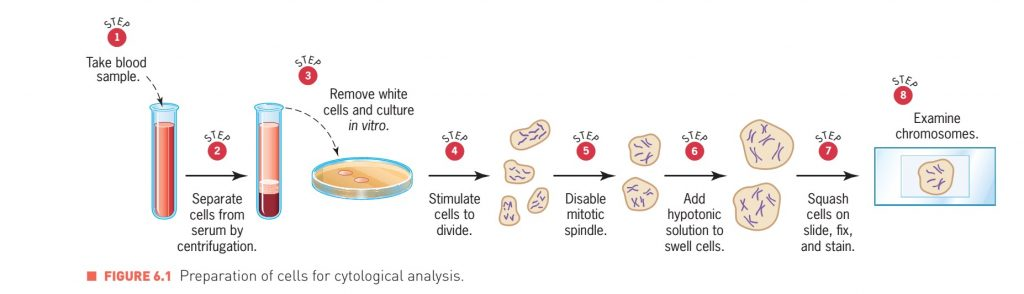
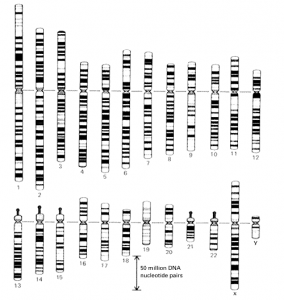
Fig. A standard map of the banding pattern of each chromosome in the human karyotype.
- If more detail is desired, the chromosomes can be treated with various enzymes in combination with stains to yield banding patterns on each chromosome.
- These techniques have become common place and will yield far more diagnostic information than Giemsa stain alone (the most commonly used process).
- A band is an area of a chromosome which is clearly distinct from its neighboring area, but may be lighter or darker than its neighboring region.
- The standard methods of banding are the Q, G, R, and C banding techniques.
- These are defined as follows
G-Banding
- This is so called because of the use of Giemsa stain.
- The bands stained with Giemsa were designated G bands.
- This is a simple two step process which involves a pretreatment with proteolytic enzyme Trypsin followed by staining with the stain which may be either Giemsa, Leishman’s stain or Wright stain. Therefore the process is also known as GTG banding (G-banding by trypsin with Giemsa)
- Pretreatment involves any treatments which remove or damage the protein content but not the DNA of the chromosome.
- Giemsa is a complex stain based on methylene blue and eosin, it is a reaction that stains the chromosome.
- Giemsa stain has more affinity for DNA sequence rich in AT content hence stained dark while sequence rich in GC content stain light.
Q- Banding
- Q Banding utilizes quinacrine dihydrochloride stain and so named.
- This intercolates between the DNA and fluoresces when UV light falls on it under fluorescence microscope, and shows a specific banding pattern
- The AT-rich regions enhance the fluorescence while GC-rich regions quench the fluorescence
- Identification of individual chromosomes can be made 1st time by the use of fluorescent Q-banding which produces similar banding pattern that of G-bands except it use fluorescent dyes, like proflavine I quinacrine and especially an alkylating derivative, quinacrine mustard.
- This banding led to the discovery of a sub-structure along the length of the chromosomes.
- Some of the alternating stains are acridine orange, EtBr, propidium iodide and Hoechst 33258 and chemically most of these are based on structure of multiple benzene rings.
- Multiple fluorochromes can be used simultaneously to increase the apparent contract which in turn enhance the banding pattern and so the result.
- The major advantage of using fluorochromes for staining is that the bonding between dye and DNA is electrostatic and therefore is easily reversible.
- So the DNA sample can be restained for more accurate results.
- Other advantages are that these stains are relatively less toxic so can be used in cell culture.
- They are more sensitive so can detect DNA even if concentration may be very low.
Fluorochrome banding has some drawbacks –
- High resolution Q-Banding is not practicable because sharp image cannot be produced.
- High cost of equipment required for fluorescent microscopy, as UV light bleached the fluorochromes.
- Toxic nature of fluorochromes, so handling required great care.
Both Q and G bands correlate with the centromeres observed in leptotene and pachytene chromosomes during meiosis. Q and G banding pattern are generally similar and correspond to the intercalary heterochromatin.
C-Banding
- This banding is used to identify the regions of constitutive heterochromatin which is genetically inert highly repetitive DNA sequences like satellite DNA.
- C-Banding is specific for species as well as individual.
- It is also a two step process i.e. pretreatment followed by staining.
- Pretreatment involves treatment with hydrochloric acid followed by either NaOH or Barium hydroxide treatment, and then incubation in a warm salt wash, and then finally Giemsa staining.
- In this, HCl depurinates the DNA without breaking the sugar –PO42-bonds.
- This is stopped before the degradation of the entire DNA.
- Then by denaturation, the alkali treatment helps in solubilization of DNA which is further aided by warm salt wash treatment which breaks the sugar-phosphate backbone.
R-Banding
- R-banding is called Reverse chromosome banding.
- With this banding technique the band pattern produced in chromosome is reversed to the band produced by G-banding and Q-banding. ie. The dark band (AT rich region) observed in G-banding technique appears light in R-banding and vice versa.
- R-banding technique also uses Giemsa stain but before staining with Giemsa the slide is heated at 88°C in a buffer solution. Heating causes denaturation of DNA.
- Denaturation of chromosome at AT rich region occurs at faster rate resulting in loss of DNA from these regions but not from GC rich region. The GC rich region is then stained by Giemsa stain which appears stained (R band)
- G-banding is usually preferred over R-banding. However R-banding can be used for chromosome identification.
- In some cases, R-banding is a useful complement to G-banding because some small light G band can be more easily detected when they are stained by R-banding.
- R-banding is also useful for visualization of telomere sequence at the ends of chromosomes. Telomeres stained dark with R-banding while light with G-banding.
Brd U Banding
- In this method, Brd U (5-bromodeoxyuridine) , an analogue of thymidine is applied which get incorporated into chromosome during s-phase of cell cycle.
- This incorporate of Brd U changes the staining properties to UV and washes in hot buffered saline, as Brd U inhibit Giemsa staining so the areas of chromosome having Brd U is less stained as compared to other parts.
- Brd U Antibodies or other similar analogoues like bromodeoxycytidine can also be used instead of Giemsa for detection.
It has two methods –
- i) B-pulse, ii) T-pulse,
RE Banding
- As the name suggest, it involves use of restriction endonucleases for banding.
D-Banding
- Produced due to differential sensitivity of chromatin to enzyme DNase I therefore also referred as DNase I sensitivity banding.
NOR Banding
- This method was successfully used in the Cytogenetics Laboratory of the Laboratory of Pathology in Seattle, Washington, USA.
- Chromosomes are treated with silver nitrate solution which binds to the Nucleolar Organizing Regions (NOR), i.e., the secondary constrictions (stalks) of acrocentric chromosomes.
T-Banding
- T-banding is used to stain the telomeric regions of chromosomes for cytogenetic analysis.
- Telomeric (or terminal) banding was first reported by Dutrillaux, who used two types of controlled thermal denaturation followed by staining with either Giemsa or acridine orange.
- The T bands apparently represent a subset of the R bands because they are smaller that the corresponding R bands and are more strictly telomeric. (Gustashaw, 1991).
DAPI/Distamycin A Staining
- The DAPI/Distamycin ,a staining technique is useful in identifying pericentromeric breakpoints in chromosomal rearrangements and in identifying chromosomes that are too small for standard banding techniques.
- Also, DAPI/DA is the method of choice for Yqh chromsome material in suspected Y autosome translocations.
- The DAPI/distamycin,a fluorescent staining technique was first described by Schweizer, Ambros, and Andrle as a method for labeling a specific subset of C bands. (Gustashaw, 1991 ).
History of Chromosome Banding Techniques
| Stain or Banding Technique | Investigator | Year |
| Q-banding | Caspersson, Zech, Johansson | 1970 |
| G-banding (by trypsin) | Seabright | 1971 |
| G-banding (by acetic-saline) | Sumner, Evans, Buckland | 1971 |
| C-banding | Arrighi, Hsu | 1971 |
| R-banding (by heat and Giemsa) | Dutrillaux, Lejeune | 1971 |
| G-11 stain | Bobrow, Madan, Pearson | 1972 |
| Antibody bands | Dev, et al | 1972 |
| R-banding (by fluorescence) | Bobrow, Madan | 1973 |
| In vitro bands (by actinomycin D) | Shafer | 1973 |
| T-banding | Dutrillaux | 1973 |
| Replication banding | Latt | 1973 |
| Silver (NOR) stain | Howell, Denton, Diamond | 1973 |
| High resolution banding | Yunis | 1975 |
| DAPI/distamycin A stain | Schweizer, Ambros, Andrle | 1978 |
| Restriction endonuclease banding | Sahasrabuddhe, Pathak, Hsu | 1978 |






