![]()
Cell shape
The Cytoskeleton.
- One distinguishing feature that separates the eukaryotes from prokaryotes is the presence of the cytoskeleton in the former, and apparent absence of it in the latter.
- This suggests that the cytoskeleton was an important factor in eukaryote evolution.
- The function of the cytoskeleton is to maintain cellular shape, and is involved in intracellular organization, cell polarity, cell adhesion, and in some cases, motility.
- The cytoskeleton is composed of several different protein components.
- There are three general classes of cytoskeletal fibers:
- (1) microtubules,
- (2) intermediate filaments, and
- (3) actin filaments.
a) Microtubules
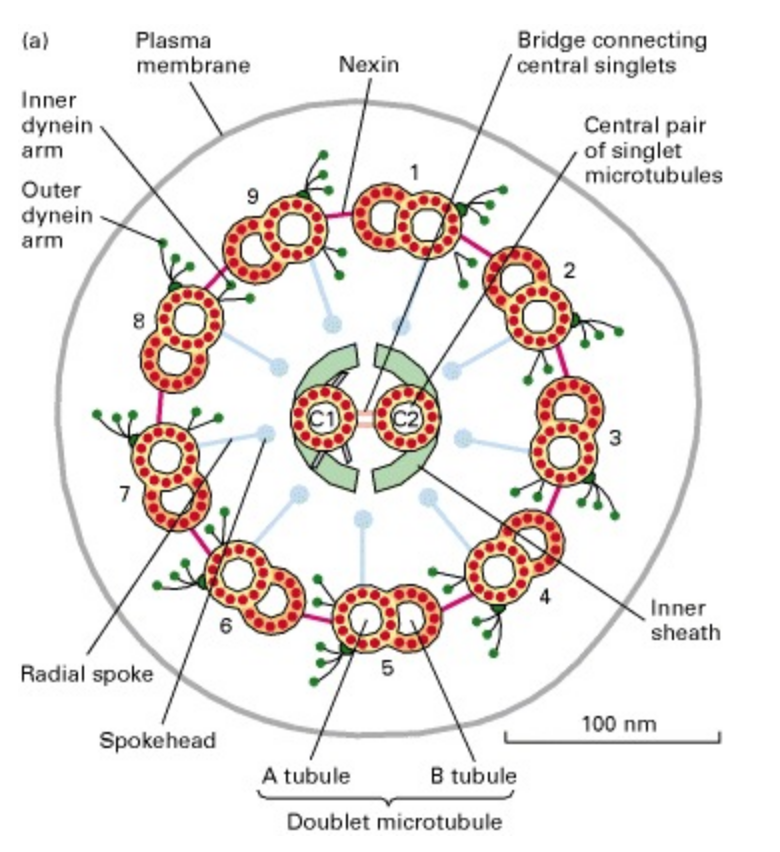
- Compared to the other cytoskeletal fibers, the microtubule is rather large (15 to 35 nm diameter).
- Microtubules are composed of a globular protein, tubulin.
- The tubulin subunit is a heterodimer of alpha- and beta-tubulin .
- The microtubule itself is made up of 13 “proto-filaments”, which are each composed of alternating alpha and beta subunits.
- These protofilaments are cylindrically arranged to form a hollow tube.
- It is the arrangement of proto-filaments that makes up the microtubule.
- Microtubules are polar molecules i.e. they have a fast growing “plus end” and a slow growing “minus end”.
- These strands are in a constant state of flux, termed “dynamic instability” (i.e. they continuously grow and fall apart).
- Free GTP (guanosine triphosphate) binds to the beta-tubulin, which alters its protein structure and enables it to join to the growing end of the strand.
- Then, there is a delayed GTP hydrolysis reaction to yield GDP (guanosine diphosphate).
- Thus, at the terminus of the filament a GTP cap is present (i.e. tubulin subunits are bound to GTP), while further down the strand, tubulin subunits are bound to GDP.
- The presence of GDP creates a weak bond between tubulin subunits, and therefore the filament is more likely to de-polymerize.
- In other words, if all the GTP is hydrolyzed in the filament, the filament is susceptible to falling apart (i.e. under a microscope, the microtubules are observed to shrink or even disappear).
- There are various proteins that are able to stabilize the microtubule, preventing de-polymerization these are known as “cap-proteins” or MAPs (Microtubule-Associated Proteins).
- There are also various other chemicals that can either stabilize or destabilize microtubules
- Another group of cytoskeletal proteins are the intermediate filaments (IFs).
- IFs have an intermediate size between microtubules and actin filaments (7 to 10 nm diameter).
- There are, however, two general types of IFs:
- (1) cytoplasmic IFs, and
- (2) nuclear lamina.
- Cytoplasmic IFs are for mechanical stress and cell-to-cell junctions.
- Nuclear lamina create a meshwork beneath the inner nuclear membrane.
- One important difference to note between IF proteins and proteins for microtubules or for microfilaments, is that most IF protein subunits are filamentous, rather than globular (i.e. tubulin and actin subunits are globular).
b) Microfilaments
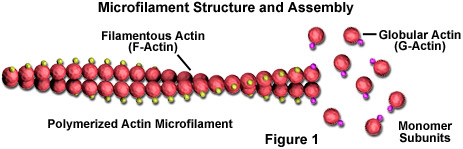
- Actin filaments (or “microfilaments” these terms are used interchangeably) are the smallest of the cytoskeletal fibers (3 to 6 nm diameter).
- Microfilaments are flexible doubled-stranded fibers composed of polymers of the protein actin (contrast this structure to microtubules, which are hollow tubes composed of 13 protofilaments).
- Actin is present in all eukaryotes, and microfilaments are typically found in the cell cortex (i.e. just beneath the cell membrane).
- The actin subunit is globular and has a molecular mass of 43 kDa.
- As with microtubules, actin filaments are also dynamic and polar molecules (i.e. they have a fast growing “plus end” and a slow growing “minus end”).
- Free actin binds ATP (adenosine-triphosphate, as opposed to GTP in tubulin subunits) which enables polymerization, while ATP hydrolysis to ADP favours depolymerization.
- Unlike microtubules which undergo dynamic instability, actin filaments may use a different dynamic process.
Actin Binding Proteins.
- The actin-based cytoskeleton functions for bearing of tension and for compression resistance (i.e. it is like a shock suspension system, which gives mechanical strength and maintains structural integrity of a cell).
- In fact, there are different microfilament arrangements for a variety of functional purposes.
- There are, however, two general classes of microfilament arrangement:
- (1) bundles (parallel and contractile), and
- (2) gel-like networks.
- Different proteins (i.e. actin-binding proteins) mediate the different arrangements.
- Parallel bundles are structures where microfilaments are oriented with the same polarity (i.e. plus ends are all “pointed” in the same direction) and which are closely spaced.
Flagella and Motility
- Most motiles move by use of flagella (flagellum) thread like locomotor appendages extending outward from the plasma membrane and cell wall.
- They are slender, rigid structures, about 20 nm across and up to 15 or 20 pm long.
- Flagella are so thin they cannot be observed directly with a bright-field microscope, but must be stained with special techniques designed to increase their thickness.
- The detailed structure of a flagellum can only be seen in the electron microscope.
- Bacterial species often differ distinctively in their patterns of flagella distribution.
- Monotrichous bacteria (trichous means hair) have one flagellum; if it is located at an end, it is said to be a polar flagellum.
- Amphitrichous bacteria (amphi mean “on both sides”) have a single flagellum at each pole.
- In contrast, lophotrichous bacteria (lopho means tuft) have a cluster of flagella at one or both ends.
- Flagella are spread fairly evenly over the whole surface of peritrichours (peri means “around”) bacteria.
- Flagellation patterns are very useful in identifying bacteria.