![]()
RNA Processing Events :
- In eukaryotes, transcription and translation take place in different cellular compartments.
- Transcription takes place in the nucleus whereas translation takes place in the cytoplasm.
- In prokaryotes, transcription of m-RNA and translation occurs simultaneously.
- Thus, mRNA molecules undergo little to no modification after synthesis by RNA polymerase in prokaryotes.
- In contrast, tRNA and rRNA in prokaryotes undergo processing like cleavage, addition of nucleotides and chemical modification after synthesis.
- Although both prokaryotes and eukaryotes modify tRNA and rRNA,
- In eukaryotes very extensively process pre-mRNA destined to become mRNA.
- The primary transcript of an RNA polymerase is referred to as pre mRNA.
- Processing of eukaryotic pre-mRNA involves the following steps:
(1) 5′ capping
(2) 3′ cleavage and polyadenylation
(3) Splicing and RNA editing.
- The conserved eukaryotic polyadenylation signal directs cleavage at the cleavage signal and addition of a poly (A) tail to the mRNA transcript.
- Post transcriptional modification or Co-transcriptional modification is a process by which in eukaryotic cells, primary transcript RNA is converted into mature RNA.
- This process is vital for the correct translation of the genomes of eukaryotes because the primary transcript contains both exons (coding sequences) and introns which are non-coding sequences
(A) 5′ Capping
- Capping of the pre-mRNA involves the addition of 7-methylguanosine (m/G) to the 5′ end.
- To achieve this, the terminal 5′ phosphate requires removal which is done with the aid of a phosphatase enzyme
- The enzyme guanosyl-transferase then catalyses the reaction which produces the diphosphate 5′ end.
- The diphosphate 5′ end then attacks the y-phosphorus atom of a GTP molecule in order to add the guanine residue in a 5’5′ triphosphate link.
- The enzyme (guanine N7-) methyltransferase [CAP MT ase”] transfers a methyl group from S-adenosyl methionine to the guanine ring
- This type of cap. with just the m’G in position is called a cap 0 structure.
- Cap 0 is the predominant cap in unicellular organism
- In some species, a methyl group is added to the second as well as the third nucleotide of the capped mRNA.
- In these cases, the methyl groups are added to the 2′-OH groups of the ribose sugar.
- mRNA with methyl groups on the terminal 7 guanine and the 2′-OH position of the second nucleotide at the 5′ end is known as cap 1.
- This is the predominant cap in multicellular organisms.
- Similarly, if the methyl group is present at both the second and third nucleotides then it is known as Cap 2. the primary
- The cap protects the 5′ end transcript from attack by ribonucleases.
(B) 3′ Processing – Cleavage and polyadenylation
- All eukaryotic mRNAs have a series of up to 250 adenosines at their 3¹ ends called a poly-A-tail.
- These are added to the transcript by a template independent RNA polymerase called poly (A) polymerase [PAP].
- This polymerase does not act at the extreme 3′ end of the transcript but at an internal site which is cleaved to create a new 3′ end to which the poly (A) tail is added.
- The poly (A) tail appears to have several functions:
1. Export of mature mRNA from the nucleus
2. Affect the stability of some mRNAs
3. Serve as a recognition signal for the ribosome.
- A single processing complex undertakes both the cutting (cleavage) and polyadenylation.
- The signal is needed for both cleavage and polyadenylation.
- In mammals, polyadenylation is directed by a signal sequence in the mRNA it is almost invariably 5′-AAUAAA-3′.
- This sequence is located between 10 and 30 nucleotides upstream of the polyadenylation site, which is immediately after the dinucleotide 5′ CA-3′ (cleavage signal) and is followed 10-20 nucleotides later by a GU-rich region.
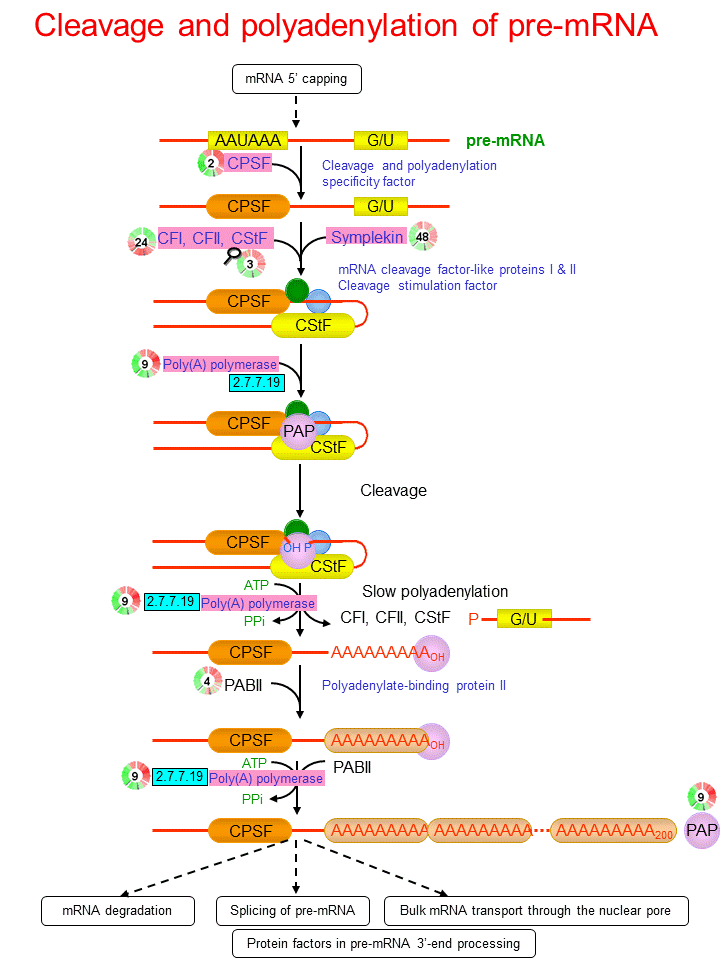
- Both the poly (A) signal and the GU-rich region are binding sites for multi subunit protein complexes, which are the cleavage and polyadenylation specificity factor (CPSF) and the cleavage stimulation factor (CSTF) respectively.
- Generation of the proper 3′ terminal structure requires an endonuclease (consisting of the components CFI, cleavage factor 1 and CF II. cleavage factor II) to cleave the RNA.
- Cleavage is followed by polyadenylation in a tightly coupled manner.
- The poly (A) polymerase has a non-specific catalytic activity.
- The polyadenylation reaction passes through two stages.
- First a short oligo (A) sequence (-10 residues) is added to the 3′ end
- In the second phase, the oligo (A) tail is extended to the full 200 residue length.
- This reaction requires another stimulatory factor that recognizes the oligo (A) tail.
- This additional factor includes polyadenylate binding protein (PADPII), which helps the polymerase to add the adenosines, possibly influences the length of the poly (A) tail that is synthesized and appears to play a role in maintenance of the tail after synthesis.
(C) Splicing
Intron Splicing:
- Non coding DNA are found within most eukaryotic genes Such genes have a split structure in which segments of coding sequence (called exons) are separated by non-coding sequences (intervening sequences or introns).
- The entire gene is transcribed to yield a large RNA molecule and the introns are then removed by splicing, so only exons are included in the mRNA.
- The process of excising the sequences in RNA that correspond to introns and joining of sequences corresponding to exons is called RNA splicing.
- Mechanism of RNA splicing varies depending on the types of introns.
- There are many types of pre m-RNA introns. Two types are commonly found in eukaryotic protein coding genes. These are
(i) The GU – AG and
(ii) AU-AC introns -.AU-AC intron is a rare class of introns.
Splicing of GU-AG intron
Self Splicing
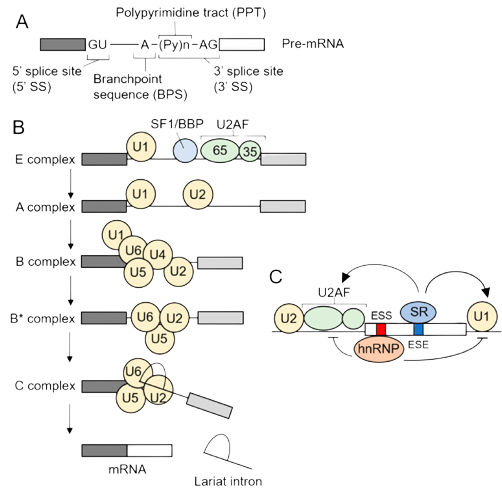
- In GU-AG intron, the first two nucleotides of the intron sequence are 5′-GU-3′ (5′ splice site or donor site) and the last two 5′-AG-3′ (3′ splice site or acceptor site).
- A pyrimidine rich region, called polypyrimidine tract, near the 3′ end of the intron is found.
- In most cases, the branch point adenosine, also invariant, usually is 20-50 bases from the 3′ splice site.
- Splicing of GU-AG intron involves two transesterification reactions.
- Cleavage of the 5′ splice site occurs by a first transesterification reaction promoted by the hydroxyl group attached to the 2′ carbon of an adenosine nucleotide located within the intron sequence.
- The result of the hydroxyl attack is cleavage of the phosphodiester bond at the 5′ splice site accompanied by the formation of a new 5-2 phosphodiester bond linking the first nucleotide of the intron (the G of the 5′-GU-3′ motif) with the internal adenosine.
- This means that the intron has now been looped back on itself to create a lariat structure.
- Cleavage of the 3′ splice site and joining of the exons result from a second transesterification reaction, this one promoted by the 3′-OH group attached to the end of upstream exon.
- This group attacks the phosphodiester bond at the 3′ splice site, cleaving it and so releasing the intron as the lariat structure, which is subsequently converted back to a linear RNA and degraded.
- At the same time, the 3′ end of the upstream exon joins to the newly formed 5′ end of the downstream exon, completing the splicing process.
- A large number of ATP is consumed during the splicing reaction.
- This energy is required for assembly of the splicing apparatus.

Splicing By Spiciosome
- The central components of the splicing apparatus for GU-AG introns are the snRNAs called UI, U2, U4, U5 and U6.
- These short RNA molecules associate with proteins to form small nuclear ribonucleoproteins (snRNPs).
- The SN RNPs. together with other accessory proteins attach to the transcript and form a series of complexes,
- last one of which is the spliceosome, the structure within which the actual splicing reactions occur.
- The process of assembly of snRNP and various protein factors occurs in the following sequence.
- (i) The E complex called commitment complex initiates a splicing activity.
- This complex comprises U1-snRNP, which binds to the 5′-splice site, the U2,AF factor which binds with the polypyrimidine tract and 3′ site.
- Binding of U1 snRNP to the 5′ splice site is the first step in splicing
- (ii) The A complex called the pre-spliceosome comprises the commitment complex plus U2-snRNP attached to the branch point.
- At this stage, an association between UI-snRNP and U2-snRNP brings the 5′ splice site into close proximity with the branch point.
- (iii) The B Complex -spliceosome is formed when U4/U6-snRNP (a single snRNP containing two snRNAs) and U5-snRNP attach to the pre-spliceosome complex.
- The B1 complex is formed when U5 and U4/U6 snRNP’s binds to the A complex.
- This complex is called spliceosome and it contains all the components needed for splicing reaction.
- It is converted to B2 complex after the UI is released.
- This catalytic reaction is triggered by the release of U4 and it requires hydrolysis of ATP
- C complex -When U4 dissociates from U6 snRNP, U6 snRNP can pair with U2 snRNP to form the catalytic active site (C complex).
- This results in additional interactions that bring the 3′ splice site close to the 5′ site and the branch point All three key positions in the intron are now in proximity and the two transesterification reactions occur as a linked reaction, catalysed by U6-snRNP, completing the splicing process.