![]()
Somatic Embryogenesis
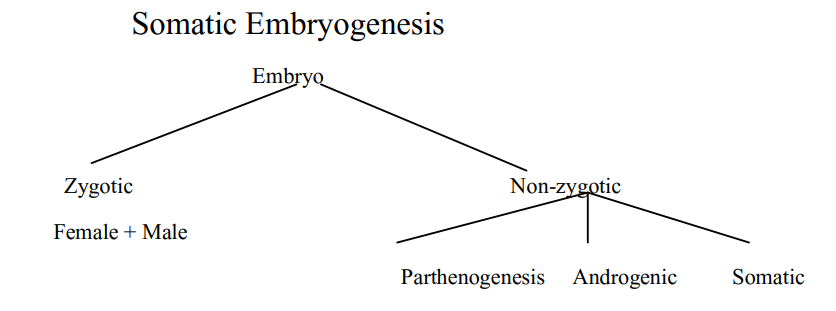
Definition: Somatic embryo (SE) is an embryo derived from a somatic cell, other than zygote, usually on culture in vitro. Somatic embryogenesis may be defined as the process of development of a bipolar structure like zygotic embryo from a non zygotic somatic cell
The embryos arise from a single cell and has no vascular connection with the maternal callus tissue on the exaplant. Induction of somatic embryogenesis require a single hormonal signal to induce a bipolar structure capable of forming a complete plant while in organogenesis it requires two different hormonal signal to induce shoot and root,
J. Reinnert (1958-1959) first reported somatic embryogenesis in Daucus carota. Capability of somatic plant cells of a culture to produce embryoids is known as embryogenic potential.
Embryogenic Potential
The capability of the somatic plant cell of a culture to produce embryoids is known as embryo genic potential.
Embryoid
Embryoid is a small, well-organised structure comparable to the sexual embryo, which is produced in tissue culture of dividing embryo genic potential somatic cells.
Somatic embryoids may be formed from:
(a) Vegetative cells of a mature plant,
(b) Reproductive cells other than zygote or
(c) Cotyledons, hypocotyl or young plantlets.
According to Sharp (’80) embryoids are formed in two ways:
(a) Directly, without callus formation, from pre-embryonic cells, i.e., the cells that are destined to form the embryo,
(b) After callus formation from induced embryo genic cells, as in carrot.
- Direct embryogenesis– The embryos initiate directly from the explant tissue in the absence of callus proliferation. This occurs through pre – embryogenic determined cells (PECD) where the cells are committed to embryonic development and need only to be released. Such cells are found in embryonic tissues. Certain tissues off young invitro grown plantlets like hypocotyl, nucellus and embryo sac.
- Indirect embryogenesis: – Cell proliferation i.e. callus from explant takes place, from which embryos are developed. The cells of embryos from which embryos arise are called embryogenically determined cells and forms embryos when induced to do so. These cells are called “induced embryogenic determined cells (IEDC) eg. secondary phloem of carrot, leaf tissues of coffee, Petunia, Asparagus etc.
In carrot root culture it has been observed that single cell should produce a cell aggregate (pro-embryo) first, before embryo initiation.
Somatic embryos arise from single cells located within a cluster of meristematic cells either in callus mass or in suspension.
Such cells develop into proembryos with polarity following a pattern that tends to mimic the general pattern associated with the development of embryos in the ovule.
Proembryo initials may be single cells or multicellular groups. When the condition are suitable these embryos germinate to produce plantlet
During initiation embryo genic cells have protoplasmic connections with adjacent cells.
Embryo genic cells have certain characteristics. These cells have dense cytoplasm, prominent nucleolus, large nucleus, conspicuous starch grains, high concentration of protein and RNA and have dehydrogenase activity. These cells are distinguished by their staining behaviour.
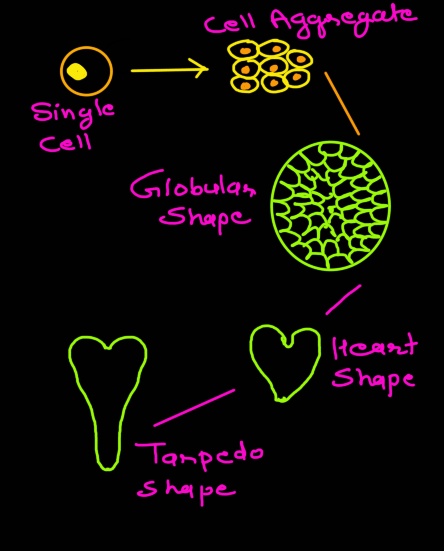
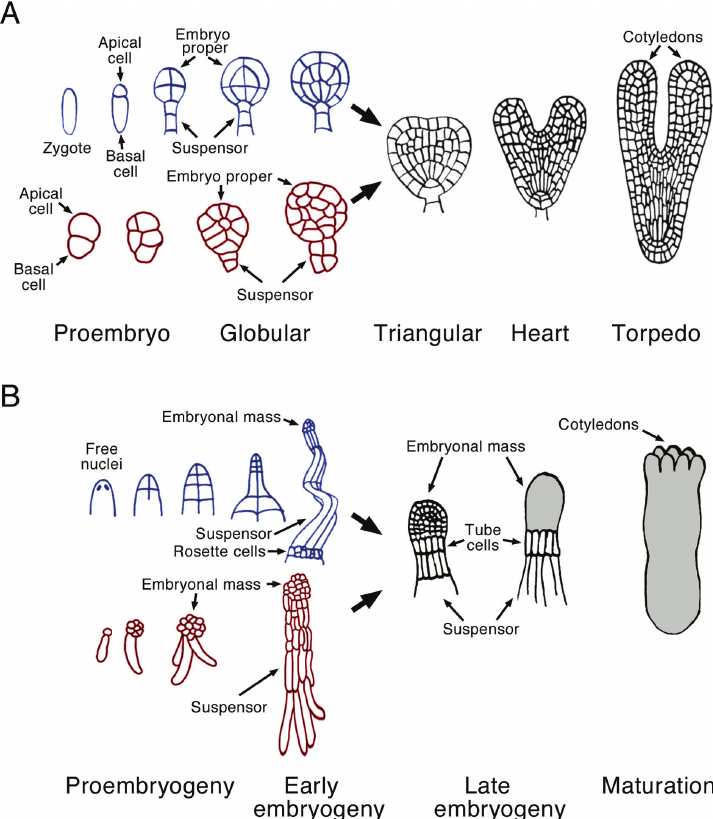
Embryoid development in tissue culture passes through three stages, namely, globular stage, heart-shaped stage and torpedo stage (Fig. 20). During embryoid formation there is first cyto-differentiation of proembryoid cells followed by occurrence of various developmental stages
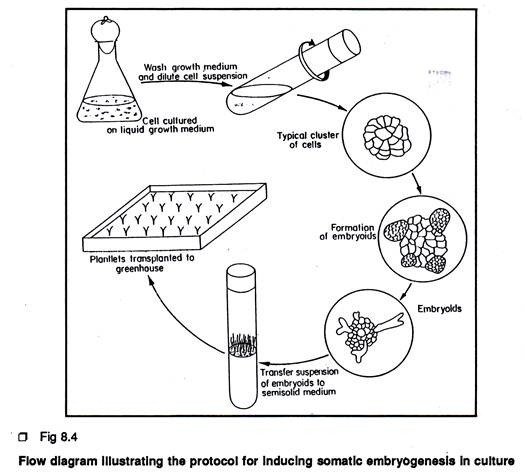
Protocols for Inducing Somatic Embryogenesis in Culture:
The plant material Daucus carota represents the classical example of somatic embryo- genesis in culture.
The protocol is described below:
1. Leaf petiole (0.5-1 cm) or root segments from seven-day old seedlings (1 cm) or cambium tissue (0.5 cm3) from storage root can be used as explant. Leaf petiole and root segment can be obtained from aseptically grown seedlings (Cambium tissue can be obtained from surface sterilized storage tap root 2. Following aseptic technique, explants are placed individually on a semi-solid Murashige and Skoog’s medium containing 0.1 mg/L 2, 4-D and 2% sucrose. Cultures are incubated in the dark. In this medium the explant will produce sufficient callus tissue.
3. After 4 weeks of callus growth, cell suspension culture is to be initiated by transferring 0.2 gm. of callus tissue to a 250 ml of Erlenmeyer flask containing 20-25 ml of liquid medium of the same composition as used for callus growth (without agar). Flasks are placed on a horizontal gyratory shaker with 125-160 rpm at 25°C. The presence or absence of light is not critical at this stage.
4. Cell suspensions are sub-cultured every 4 weeks by transferring 5 ml to 65 ml of fresh liquid medium.
5. To induce a more uniform embryo population, cell suspension is passed through a series of stainless steel mesh sieves. For carrot, the 74 µ sieve produces a fairly dense suspension of single cell and small multiple clumps. To induce somatic embryogenesis, portions of sieved cell suspension are transferred to 2, 4-D free liquid medium or cell suspension can be planted in semi-solid MS medium devoid of 2, 4-D. For normal embryo development and to inhibit precocious germination especially root elongation, 0.1- 1 µM ABA can be added to the culture medium. Cultures are incubated in dark.
6. After 3-4 weeks, the culture would contain numerous embryos in different stages of development.
7. Somatic embryos can be placed on agar medium devoid of 2, 4-D for plantlet development.
8. Plantlets are finally transferred to Jiffy pots or vermiculite for subsequent development.
Methods of embryogenesis from cell suspension culture of carrot root:
Carrot roots are cultured and callus is obtained. From an actively growing callus explants are taken for suspension culture on Murashige and Skoog’s medium supplemented with sucrose, 0.2 mg/1 zeatin and 1 % agar.
On this auxin free medium embryoids are formed. After the embryoids have reached the torpedo stage, they are transferred to filter paper bridges on tubes containing Murashige and Skoog’s medium with 2% sucrose and 0.2 mg/1 kinetin. Plantlets formed are later transferred to soil under humid condition.
Importance of Somatic Embryogenesis:
The potential applications and importance of in vitro somatic embryogenesis and organogenesis are more or less similar. The mass production of adventitious embryos in cell culture is still regarded by many as the ideal propagation system. The adventitious embryo is a bipolar structure that develops directly into a complete plantlet and there is no need for a separate rooting phase as with shoot culture.
Somatic embryo has no food reserves, but suitable nutrients could be packaged by coating or encapsulation to form some kind of artificial seeds. Such artificial seeds produce the plantlets directly into the field. Unlike organogenesis, somatic embryos may arise from single cells and so it is of special significance in mutagenic studies.
Plants derived from asexual embryos may in some cases be free of viral and other pathogens. For an example, Citrus plant propagation from embryo genic callus of nuclear origin are free of Virus. So it is an alternative approach for the production of disease-free plants.
Factors influencing somatic embryogenesis:
(1) Auxin:
In medium having relatively high concentration of auxin embryonal budding or embryonal clumps have been observed. For cell differentiation the medium should contain auxin and reduced nitrogen.
Subsequent development takes place in medium with no auxin or low concentration of auxin and reduced nitrogen. In some plants first and second stages occur in the first medium and plantlet development takes place in the second medium.
(2) Nitrogen:
The ratio of nitrogen to auxin is an important factor controlling embryogenesis. Embryo development can be initiated on White’s medium with low nitrogen content only in absence of auxin. At low nitrogen concentration organic nitrogen is more suitable than inorganic nitrogen.
Substances used as a source of nitrogen are potassium nitrate, ammonium chloride, glutamine, glutamic acid, alanine, urea etc. Endogenous polyamines, such as, putrescine, sperdimine or spermine are required for induction of embryogenesis in wild carrot culture.
(3) Cytokinin:
The role of cytokinin in embryogenesis is not clear. Embyogenesis in carrot cell suspension is stimulated by addition of zeatin in medium lacking auxin but inhibited by the addition of kinetin. Inhibitory effect of exogenous cytokinin may be due to an increase in endogenous cytokinin in growing embryoids.
(4) Activated charcoal:
Presence of activated charcoal in the medium helps embryogenesis in several cases, as in Daucas carota, Hedera helix etc. Activated charcoal may adsorb the inhibitory substances present in the medium.
(5) Age of the culture:
Embryogenesis usually occurs in short-term cultures. With older cultures this ability decreases and ultimately it is completely lost. This may be due to either the inability to synthesise some embryogenetic substances or changes in the ploidy level which may lead to loss of morphogenetic potential.
In carrot culture embryoid formation starts 4-6 weeks after isolation of the tissue. The, optimal embryo genetic potential is reached after 15 weeks, then this potentiality gradually decreases and ultimately lost after 36 weeks. But carrot tissue may regain the embryo genetic potentiality when transferred to a medium containing proper nutrient substances.
Other Factors:
The medium supplemented with activated charcoal has facilitated embryogenesis in several culture. The induction of embryogenesis is achieved successfully by the addition of charcoal when auxin depletion in the medium fails to produce the desired results.
It has been suggested that charcoal may absorb a wide variety of inhibitory substances as well as hormone. Optimal level of dissolved oxygen and high potassium in the medium are necessary for embryogenesis. But in Citrus, certain volatile and non-volatile substances inhibit embryogenesis.
Significance:
By somatic embryogenesis many plants are produced very rapidly. By this method a desirable plant can be multiplied very quickly.