![]()
Clonal Propagation (Micro propagation)
Micro Propagation:
Plants can be propagated by sexual (through generation of seeds) or asexual (through multiplication of vegetative parts) means.
Clonal propagation refers to the process of asexual reproduction by multiplication of genetically identical copies of individual plants. The term clone is used to represent a plant population derived from a single individual by asexual reproduction.
Asexual reproduction through multiplication of vegetative parts is the only method for the in vivo propagation of certain plants, as they do not produce viable seeds e.g. banana, grape, fig, and chrysanthemum. Clonal propagation has been successfully applied for the propagation of apple, potato, tuberous and several ornamental plants.
Advantages of Vegetative Propagation:
Asexual (vegetative) propagation of plants has certain advantages over sexual propagation.
i. Faster multiplication — large number of plants can be produced from a single individual in a short period.
ii. Possible to produce genetically identical plants.
iii. Sexually — derived sterile hybrids can be propagated.
iv. Seed — raised plants pass through an undesirable juvenile phase which is avoided in asexual propagation.
v. Gene banks can be more easily established by clonally propagated plants.
In Vitro Clonal Propagation:
The in vivo clonal propagation of plants is tedious, expensive and frequently unsuccessful. In vitro clonal propagation through tissue culture is referred to as micro propagation. Use of tissue culture technique for micro propagation was first started by Morel (1960) for propagation of orchids, and is now applied to several plants. Micro propagation is a handy technique for rapid multiplication of plants.
Technique of Micro propagation:
Micro propagation is a complicated process and mainly involves 3 stages (I, II and III). Some authors add two more stages (stage 0 and IV) for more comprehensive representation of micro- propagation. All these stages are represented in Fig. 47.1, and briefly described hereunder.

Stage 0:
This is the initial step in micro- propagation, and involves the selection and growth of stock plants for about 3 months under controlled conditions.
Stage I:
In this stage, the initiation and establishment of culture in a suitable medium is achieved. Selection of appropriate explants is important. The most commonly used explants are organs, shoot tips and axillary buds. The chosen explant is surface sterilized and washed before use.
Stage II:
It is in this stage, the major activity of micro propagation occurs in a defined culture medium. Stage II mainly involves multiplication of shoots or rapid embryo formation from the explant.
Stage III:
This stage involves the transfer of shoots to a medium for rapid development into shoots. Sometimes, the shoots are directly planted in soil to develop roots. In vitro rooting of shoots is preferred while simultaneously handling a large number of species.
Stage IV:
This stage involves the establishment of plantlets in soil. This is done by transferring the plantlets of stage III from the laboratory to the environment of greenhouse. For some plant species, stage III is skipped, and un-rooted stage II shoots are planted in pots or in suitable compost mixture.
The different stages described above for micro propagation are particularly useful for comparison between two or more plant systems, besides better understanding. It may however, be noted that not all plant species need to be propagated in vitro through all the five stages referred above.
Micro propagation mostly involves in vitro clonal propagation by two approaches:
1. Multiplication by axillary buds/apical shoots.
2. Multiplication by adventitious shoots.
Besides the above two approaches, the plant regeneration processes namely organogenesis and somatic embryogenesis may also be treated as micro propagation.
3. Organogenesis: The formation of individual organs such as shoots, roots, directly from an explant (lacking preformed meristem) or from the callus and cell culture induced from the explant.
4. Somatic embryogenesis: The regeneration of embryos from somatic cells, tissues or organs.
1. Multiplication by Axillary Buds and Apical Shoots:
Quiescent or actively dividing meristems are present at the axillary and apical shoots (shoot tips). The axillary buds located in the axils of leaves are capable of developing into shoots. In the in vivo state, however only a limited number of axillary meristems can form shoots. By means of induced in vitro multiplication in micro propagation, it is possible to develop plants from meristem and shoot tip cultures and from bud cultures.
Meristem and Shoot Tip Cultures:
Apical meristem is a dome of tissue located at the extreme tip of a shoot. The apical meristem along with the young leaf primordia constitutes the shoot apex. For the development of disease-free plants, meristem tips should be cultured.
Meristem or shoot tip is isolated from a stem by a V-shaped cut. The size (frequently 0.2 to 0.5 mm) of the tip is critical for culture. In general, the larger the explant (shoot tip), the better are the chances for culture survival. For good results of micro propagation, explants should be taken from the actively growing shoot tips, and the ideal timing is at the end of the plants dormancy period.
The most widely used media for meristem culture are MS medium and White’s medium. A diagrammatic representation of shoot tip (or meristem) culture in micro propagation is given in Fig 47.2, and briefly described hereunder.

In stage I, the culture of meristem is established. Addition of growth regulators namely cytokinins (kinetin, BA) and auxins (NAA or IBA) will support the growth and development.
In stage II, shoot development along with axillary shoot proliferation occurs. High levels of cytokinins are required for this purpose.
Stage III is associated with rooting of shoots and further growth of plantlet. The root formation is facilitated by low cytokinin and high auxin concentration. This is opposite to shoot formation since high level of cytokinins is required (in stage II). Consequently, stage II medium and stage III medium should be different in composition. The optimal temperature for culture is in the range of 20-28°C (for majority 24-26°C). Lower light intensity is more appropriate for good micro propagation.
Bud Cultures:
The plant buds possess quiescent or active meristems depending on the physiological state of the plant. Two types of bud cultures are used— single node culture and axillary bud culture.
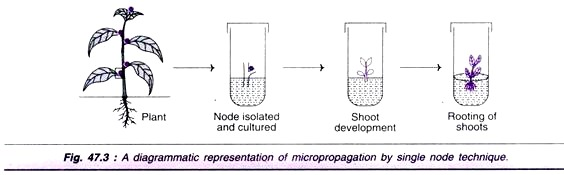
Single node culture:
This is a natural method for vegetative propagation of plants both in vivo and in vitro conditions. The bud found in the axil of leaf is comparable to the stem tip, for its ability in micro propagation. A bud along with a piece of stem is isolated and cultured to develop into a plantlet. Closed buds are used to reduce the chances of infections.
A diagrammatic representation of single node culture is depicted in Fig 47.3. In single node culture, no cytokinin is added.
Axillary bud culture:
In this method, a shoot tip along with axillary bud is isolated. The cultures are carried out with high cytokinin concentration. As a result of this, apical dominance stops and axillary buds develop. A schematic representation of axillary bud culture for a rosette plant and an elongate plant is given in Fig 47.4.

For a good axillary bud culture, the cytokinin/ auxin ratio is around 10: 1. This is however, variable and depends on the nature of the plant species and the developmental stage of the explant used. In general, juvenile explants require less cytokinin compared to adult explants. Sometimes, the presence of apical meristem may interfere with axillary shoot development. In such a case, it has to be removed.
2. Multiplication by Adventitious Shoots:
The stem and leaf structures that are naturally formed on plant tissues located in sites other than the normal leaf axil regions are regarded as adventitious shoots. There are many adventitious shoots which include stems, bulbs, tubers and rhizomes. The adventitious shoots are useful for in vivo and in vitro clonal propagation. The meristematic regions of adventitious shoots can be induced in a suitable medium to regenerate to plants.
3. Organogenesis:
Organogenesis is the process of morphogenesis involving the formation of plant organs i.e. shoots, roots, flowers, buds from explant or cultured plant tissues. It is of two types — direct organogenesis and indirect organogenesis.
Direct Organogenesis:
Tissues from leaves, stems, roots and inflorescences can be directly cultured to produce plant organs. In direct organogenesis, the tissue undergoes morphogenesis without going through a callus or suspension cell culture stage. The term direct adventitious organ formation is also used for direct organogenesis.
Induction of adventitious shoot formation directly on roots, leaves and various other organs of intact plants is a widely used method for plant propagation. This approach is particularly useful for herbaceous species. For appropriate organogenesis in culture system, exogenous addition of growth regulators—auxin and cytokinin is required. The concentration of the growth promoting substance depends on the age and nature of the explant, besides the growth conditions.
Indirect Organogenesis:
When the organogenesis occurs through callus or suspension cell culture formation, it is regarded as indirect organogenesis (Fig 47.5 B and C). Callus growth can be established from many explants (leaves, roots, cotyledons, stems, flower petals etc.) for subsequent organogenesis.

The explants for good organogenesis should be mitotically active immature tissues. In general, the bigger the explant the better the chances for obtaining viable callus/cell suspension cultures. It is advantageous to select meristematic tissues (shoot tip, leaf, and petiole) for efficient indirect organogenesis. This is because their growth rate and survival rate are much better.
For indirect organogenesis, the cultures may be grown in liquid medium or solid medium. Many culture media (MS, B5 White’s etc.) can be used in organogenesis. The concentration of growth regulators in the medium is critical for organogenesis.
By varying the concentrations of auxins and cytokinins, in vitro organogenesis can be manipulated:
i. Low auxin and low cytokinin concentration will induce callus formation.
ii. Low auxin and high cytokinin concentration will promote shoot organogenesis from callus.
iii. High auxin and low cytokinin concentration will induce root formation.
4. Somatic Embryogenesis:
The process of regeneration of embryos from somatic cells, tissues or organs is regarded as somatic (or asexual) embryogenesis. Somatic embryogenesis may result in non-zygotic embryos or somatic embryos (directly formed from somatic organs), parthogenetic embryos (formed from unfertilized egg) and androgenic embryos (formed from male gametophyte).
In a general usage, when the term somatic embryo is used it implies that it is formed from somatic tissues under in vitro conditions. Somatic embryos are structurally similar to zygotic (sexually formed) embryos, and they can be excised from the parent tissues and induced to germinate in tissue culture media.
Development of somatic embryos can be done in plant cultures using somatic cells, particularly epidermis, parenchymatous cells of petioles or secondary root phloem. Somatic embryos arise from single cells located within the clusters of meristematic cells in the callus or cell suspension. First a pro-embryo is formed which then develops into an embryo, and finally a plant.
Two routes of somatic embryogenesis are known — direct and indirect (Fig 47.6).

Direct Somatic Embryogenesis:
When the somatic embryos develop directly on the excised plant (explant) without undergoing callus formation, it is referred to as direct somatic embryogenesis (Fig 47.6A). This is possible due to the presence of pre-embryonic determined cells (PEDQ found in certain tissues of plants. The characteristic features of direct somatic embryogenesis is avoiding the possibility of introducing somaclonal variations in the propagated plants.
Indirect Somatic Embryogenesis:
In indirect embryogenesis, the cells from explant (excised plant tissues) are made to proliferate and form callus, from which cell suspension cultures can be raised. Certain cells referred to as induced embryo genic determined cells (IEDC) from the cell suspension can form somatic embryos. Embryogenesis is made possible by the presence of growth regulators (in appropriate concentration) and under suitable environmental conditions.
Somatic embryogenesis (direct or indirect) can be carried on a wide range of media (e.g. MS, White’s). The addition of the amino acid L-glutamine promotes embryogenesis. The presence of auxin such as 2, 4-dichlorophenoxy acetic acid is essential for embryo initiation. On a low auxin or no auxin medium, the embryo genic clumps develop into mature embryos.
Indirect somatic embryogenesis is commercially very attractive since a large number of embryos can be generated in a small volume of culture medium. The somatic embryos so formed are synchronous and with good regeneration capability.
Artificial Seeds from Somatic Embryos:
Artificial seeds can be made by encapsulation of somatic embryos. The embryos, coated with sodium alginate and nutrient solution, are dipped in calcium chloride solution. The calcium ions induce rapid cross-linking of sodium alginate to produce small gel beads, each containing an encapsulated embryo. These artificial seeds (encapsulated embryos) can be maintained in a viable state till they are planted.
Factors Affecting Micro propagation:
For a successful in vitro clonal propagation (micro propagation), optimization of several factors is needed.
Some of these factors are briefly described:
1. Genotype of the plant:
Selection of the right genotype of the plant species (by screening) is necessary for improved micro propagation. In general, plants with vigorous germination and branching capacity are more suitable for micro- propagation.
2. Physiological status of the explants:
Explants (plant materials) from more recently produced parts of plants are more effective than those from older regions. Good knowledge of donor plants’ natural propagation process with special reference to growth stage and seasonal influence will be useful in selecting explants.
3. Culture media:
The standard plant tissue culture media are suitable for micro propagation during stage I and stage II. However, for stage III, certain modifications are required. Addition of growth regulators (auxins and cytokinins) and alterations in mineral composition are required. This is largely dependent on the type of culture (meristem, bud etc.).
4. Culture environment:
Light:
Photosynthetic pigment in cultured tissues does absorb light and thus influence micro- propagation. The quality of light is also known to influence in vitro growth of shoots, e.g blue light induced bud formation in tobacco shoots. Variations in diurnal illumination also influence micro propagation. In general, an illumination of 16 hours day and 8 hours night is satisfactory for shoot proliferation.
Temperature:
Majority of the culture for micro propagation requires an optimal temperature around 25°C. There are however, some exceptions e.g. Begonia X Cheimantha hybrid tissue grows at a low temperature (around 18°C).
Composition of gas phase:
The constitution of the gas phase in the culture vessels also influences micro propagation. Unorganized growth of cells is generally promoted by ethylene, O2, CO2 ethanol and acetaldehyde.
Factors Affecting in Vitro Rooting:
A general description of the factors affecting micro propagation, particularly in relation to shoot multiplication is given above. For efficient in vitro rooting during micro- propagation, low concentration of salts (reduction to half to one quarter from the original) is advantageous. Induction of roots is also promoted by the presence of suitable auxin (NAA or IBA).
Applications of Micro propagation:
Micro propagation has become a suitable alternative to conventional methods of vegetative propagation of plants. There are several advantages of micro propagation.
High Rate of Plant Propagation:
Through micro propagation, a large number of plants can be grown from a piece of plant tissue within a short period. Another advantage is that micro propagation can be carried out throughout the year, irrespective of the seasonal variations. Further, for many plants that are highly resistant to conventional propagation, micro propagation is the suitable alternative. The small sized propagules obtained in micro propagation can be easily stored for many years (germplasm storage), and transported across international boundaries.
Production of Disease-free Plants:
It is possible to produce disease-free plants through micro propagation. Meristem tip cultures are generally employed to develop pathogen-free plants .In fact, micro propagation is successfully used for the production of virus-free plants of sweet potato (Ipomea batatus), cassava (Manihot esculenta) and yam (Discorea rotundata).
Production of Seeds in Some Crops:
Micro propagation, through axillary bud proliferation method, is suitable for seed production in some plants. This is required in certain plants where the limitation for’ seed production is high degree of genetic conservation e.g. cauliflower, onion.
Cost-effective Process:
Micro propagation requires minimum growing space. Thus, millions of plant species can be maintained inside culture vials in a small room in a nursery. The production cost is relatively low particularly in developing countries (like India) where the manpower and labour charges are low.
Automated Micro propagation:
It has now become possible to automate micro propagation at various stages. In fact, bio- reactors have been set up for large scale multiplication of shoots and bulbs. Some workers employ robots (in place of labourers) for micro- propagation, and this further reduces production cost of plants.
Disadvantages of Micro propagation:
Contamination of Cultures:
During the course of micro propagation, several slow-growing microorganisms (e.g. Eswinia sp, Bacillus sp) contaminate and grow in cultures. The microbial infection can be controlled by addition of antibiotics or fungicides. However, this will adversely influence propagation of plants.
Brewing of Medium:
Micro propagation of certain plants (e.g. woody perennials) is often associated with accumulation of growth inhibitory substances in the medium. Chemically, these substances are phenolic compounds, which can turn the medium into dark colour. Phenolic compounds are toxic and can inhibit the growth of tissues. Brewing of the medium can be prevented by the addition of ascorbic acid or citric acid or polyvinyl pyrrolidone to the medium.
Genetic Variability:
When micro propagation is carried out through shoot tip cultures, genetic variability is very low. However, use of adventitious shoots is often associated with pronounced genetic variability.
Vitrification:
During the course of repeated in vitro shoot multiplication, the cultures exhibit water soaked or almost translucent leaves. Such shoots cannot grow and even may die. This phenomenon is referred to as vitrification. Vitrification may be prevented by increasing the agar concentration (from 0.6 to 1%) in the medium. However, increased agar concentration reduces the growth rate of tissues.
Cost Factor:
For some micro propagation techniques, expensive equipment, sophisticated facilities and trained manpower are needed. This limits its use.