Chemical and Physical Mutagens
1. Chemical Mutagens:
These are the following types
1. Base Analogues:
- A base analogue is a chemical compound similar to one of the four bases of DNA.
- It can be incorporated into a growing polynucleotide chain when normal process of replication occurs.’
- These compounds have base pairing properties different from the bases.
- They replace the bases and cause stable mutation.
- A very common and widely used base analogue is 5-bromouracil (5-BU) which is an analogue of thymine.
- The 5-BU functions like thymine and pairs with adenine (Fig. 9.6A).
- The 5-BU undergoes tautomeric shift from keto form to enol form caused by bromine atom.
- The enol form can exist for a long time for 5-BU than for thymine (Fig. 9.6B).
- If 5-BU replaces a thymine, it generates a guanine during replication which in turn specifies cytosine causing G: C pair (Fig. 9.6A).

- During the replication, keto form of 5-BU substitutes for T and the replication of an initial AT pair becomes an A: BU pair (Fig. 9.7A).
- The rare enol form of 5-BU that pairs with G is the first mutagenic step of replication. In the next round of replication G pairs with C.
- Thus, the transition is completed from AT→GC pair.
- The 5-BU can also induce the conversion of GC to AT.
- The enol form infrequently acts as an analogue of cytosine rather than thymine. Due to error, GC pair is converted into a G: BU pair which in turn becomes an AT pair (Fig. 9.7B).
- Due to such pairing properties 5-BU is used in chemotherapy of viruses and cancer.
- Because of pairing with guanine it disturbs the normal replication process in microorganisms.

- The 5-bromodeoxyuridine (5-BDU) can replace thymidine in DNA molecule.
- The 2-amino-purine (2-AP) and 2, 6-di-amino-purine (2, 6-DAP) are the purine analogues.
- The 2-AP normally pairs with thymine but it is able to form a single hydrogen bond with cytosine resulting in transition of AT to GC.
- The 2-AP and 2, 6-DAP are not as effective as 5-BU and 5-BDU.
2. Chemicals Changing the Specificity of Hydrogen Bonding:
- There are many chemicals that after incorporation into DNA change the specificity of hydrogen -bonding.
- Those which are used as mutagens are nitrous oxide (HNO2), hydroxylamine (HA) and ethyl-methane-sulphonate (EMS).
(a) Nitrous Oxide (HNO2):
- Nitrous oxide converts the amino group of bases into keto group through oxidative deamination. The order of frequency of deamination (removal of amino group) is adenine > cytosine > guanine.
(b) Deamination of Adenine:
- Deamination of adenine results in formation of hypoxanthine, the pairing behaviour of which is like guanine.
- Hence, it pairs with cytosine instead of thymine replacing AT pairing by GC pairing (Fig. 9.8A).

(c) Deamination of Cytosine:
- Deamination of cytosine results in formation of uracil by replacing – NH2 group with -OH group.
- The affinity for hydrogen bonding of uracil is like thymine; therefore, C-G pairing is replaced by U-A pairing (Fig. 9.8B).
(d) Deamination of Guanine:
- Deamination of guanine results in formation of xanthine, the later is not mutagenic. Xanthine behaves like guanine because there is no change in pairing behaviour. Xanthine pairs with cytosine.
- Therefore, G-C pairing is replaced by X-C pairing.
(e) Hydroxylamine (NH2OH):
- It hydroxylates the C4 nitrogen of cytosine and converts into a modified base via deamination which causes to base pairs like thyamine.
- Therefore, GC pairs are changed into AT pairs.
3. Alkylating Agents:
- Addition of an alkyl group to the hydrogen bonding oxygen of guanine (N7 position) and adenine (at N3 position) residues of DNA is done by alkylating agents.
- As a result of alkylation, possibility of ionization is increased with the introduction of pairing errors.
- Hydrolysis of linkage of base-sugar occurs resulting in gap in one chain.
- This phenomenon of loss of alkylated base from the DNA molecule (by breakage of bond joining the nitrogen of purine and deoxyribose) is called depurination.
- Depurination is not always mutagenic. The gap created by loss of a purine can effectively be repaired.
Following are some of the important widely used alkylating agents:
(a) Dimethyl sulphate (DMS)
(b) Ethyl methane sulphonate (EMS) -CH3CH2SO3CH3
(c) Ethyl ethane sulphonate (EES) -CH3CH2SO3CH2CH3
- EMS has the specifity to remove guanine and cytosine from the chain and results in gap formation. Any base (A,T,G,C) may be inserted in the gap.
- During replication chain without gap will result in normal DNA. In the second round of replication gap is filled by suitable base.
- If the correct base is inserted, normal DNA sequence will be produced. Insertion of incorrect bases results in transversion or transition mutation.
- Another example is methyl nitrosoguanidine that adds methyl group to guanine causing it to mispair with thyamine. After subsequent replication, GC is converted into AT transition.
4. Intercalating Agents:
- There are certain dyes such as acridine orange, proflavine and acriflavin which are three ringed molecules of similar dimensions as those of purine pyrimidine pairs (Fig. 9.9).
- In aqueous solution these dyes can insert themselves in DNA (i.e. intercalate the DNA) between the bases in adjacent pairs by a process called intercalation.
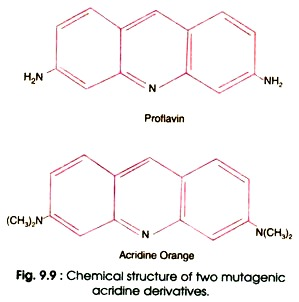
- Therefore, the dyes are called intercalating agents.
- The acridines are planer (flat) molecules which can be intercalated between the base pairs of DNA; distort the DNA and results deletion or insertion after replication of DNA molecule.
- Due to deletion or insertion of intercalating agents, there occur frameshift mutations (Fig. 9.10).

2. Physical Mutagens:
Radiations as Mutagens:
- Radiation is the most important among the physical mutagens. Radiations damaging the DNA molecules fall in the wavelength range below 340 nm and photon energy above 1 electro-volt (eV).
- The destructive radiation consists of ultraviolet (UV) rays, X-rays, ү-rays, alpha (α) rays, beta (β) rays, cosmic rays, neutrons, etc. (Fig. 9.11).

- Radiation induced damage can be categorized into the three broad types: lethal damage (killing the organisms), potentially lethal damage (can be lethal under certain ordinary conditions) and sub-lethal damage (cells do not die unless radiation reaches to a certain threshold value).
- The effect of damage is at molecular level.
- In a live cell radiation damage to proteins, lipoproteins, DNA, carbohydrates, etc. is caused directly by ionization/excitation, or indirectly through highly reactive free radicals produced by radiolysis of cellular water.
- DNA stores genetic information’s so a damage to it assumes great dimension.
- It can perpetuate genetic effects and, therefore, the cellular repair system is largely devoted to its welfare.
- When the bacteria are exposed to radiation they gradually lose the ability to develop colonies.
- This gradual loss of viability can be expressed graphically by plotting the surviving colonies against the gradually increasing exposure time.
- This dose-response graph is called survival curve.
- The survival curve of bacteria is given in Fig. 9.12.
- The survival curve is analysed by a simple mathematical theory called hit theory.

Ultraviolet (UV) Radiation:
- UV radiation causes damage in the DNA duplex of the bacteria and phages.
- The UV rays are absorbed and cause excitation of macromolecules.
- The absorption maxima of nucleic acid = (280 nm) and protein (260 nm) are more or less similar.
- The DNA molecule is the target molecule for UV rays but not the proteins. However, absorption spectrum of RNA is quite similar to that of DNA.
- The excited DNA leads to cross-linking, single strand breaks and base damage as minor lesion and generation of nucleotide dimer as a major one.
- Purines are generally more radio – resistant than the pyrimidine of the latter, thymine is more reactive than cytosine.
- Hence, the ratio of thymine-thymine (TT), thymine-cytosine (TC), cytosine-cytosine (CC) dimer (Fig. 9.14) is 10:3:3, respectively.
- A few dimers of TU and UU also appear.
- The initial step in pyrimidine dimerization is known to be hydration of their 4: 5 bonds.

- Formation of thymine-thymine (TT) dimer causes distortion of DNA helix because the thymines are pulled towards one another.
- The distortion results in weakening of hydrogen-bonding to adenines in the opposing strand.
- This structural distortion inhibits the advance of replication fork.
The X-Rays:
- The X-rays cause breaking of phosphate ester linkages in the DNA.
- This breakage occurs at one or more points. Consequently, a large number of bases are deleted or rearranged in the DNA molecule.
- The X-rays may break the DNA either in one or both strands.
- If breaks occur in both strands, it becomes lethal.
- The DNA segment between the two breaks is removed resulting in deletion.
- Since both the X-rays and UV rays bring about damage in DNA molecule, they are used in sterilization of bacteria and viruses.


