Isolation of Bacteria
Introduction
Isolation of bacteria is a fundamental technique in microbiology in which a single species of bacterium is separated from a mixed microbial population and grown as a pure culture. Since bacteria normally occur in mixed communities in nature (soil, water, air, food, or clinical samples), isolation is essential for their study, identification, classification, and practical applications.
Definition
Isolation of bacteria is the process by which individual bacterial cells are separated from a mixed population and allowed to multiply on a suitable solid culture medium to form distinct colonies, each arising from a single cell or a group of identical cells.
Objectives of Bacterial Isolation
- To obtain pure cultures of bacteria
- To study morphological, physiological, and biochemical characters
- To identify pathogenic bacteria
- To test antibiotic sensitivity
- To use bacteria for industrial, agricultural, and research purposes
Sources of Bacteria
- Soil
- Water (freshwater and marine)
- Air and dust
- Food and dairy products
- Clinical samples (blood, urine, sputum, pus)
Methods of Isolation of Bacteria
Serial Dilution Technique
Introduction
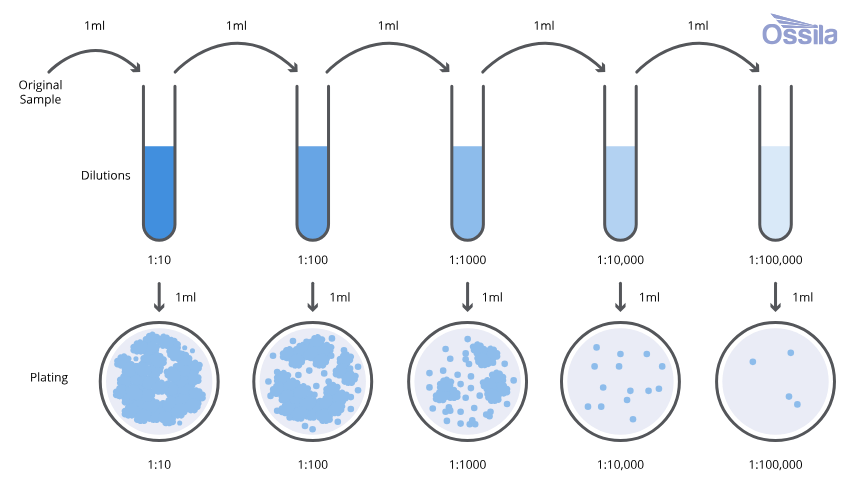
The serial dilution technique is an important microbiological method used to reduce the concentration of bacterial cells in a sample in a stepwise manner. Natural samples such as soil, water, sewage, and clinical materials usually contain a very high number of microorganisms, making direct isolation impossible. Serial dilution enables separation of individual bacterial cells so that they can be isolated as discrete colonies on solid media.
Definition
Serial dilution is a systematic process of diluting a microbial suspension step by step (usually in ten-fold dilutions) so that the number of microorganisms is reduced to a level where individual colonies can be obtained on agar plates.
Principle
The technique works on the principle of gradual reduction in microbial density. Each dilution decreases the number of bacterial cells by a fixed factor (e.g., 10 times). When diluted samples are plated, bacteria become sufficiently separated to form isolated colonies, each representing a single viable cell or clonal group.
Procedure
- A known volume of the sample is added to a sterile diluent (distilled water or saline) to make the first dilution (10⁻¹).
- The diluted sample is mixed thoroughly.
- A measured volume from this dilution is transferred to another tube containing sterile diluent to make the next dilution (10⁻²).
- This process is repeated to obtain a series of dilutions (10⁻³, 10⁻⁴, etc.).
- A suitable dilution is inoculated on agar plates using spread plate or pour plate methods.
- Plates are incubated under suitable conditions to allow colony formation.
Applications of Serial Dilution
- Isolation of bacteria from mixed populations
- Enumeration of viable bacteria (plate count method)
- Microbiological analysis of water, milk, and food
- Soil microbiology studies
- Preparation of inoculum for industrial fermentation
Advantages
- Simple and economical technique
- Effective for highly contaminated samples
- Allows quantitative estimation of bacteria
- Can be combined with other isolation methods
Limitations
- Time-consuming
- Errors in pipetting may affect accuracy
- Not suitable for bacteria that do not grow on artificial media
Precautions
- Use sterile pipettes and dilution blanks
- Mix each dilution thoroughly
- Maintain aseptic conditions
- Select appropriate dilution for plating
Significance
Serial dilution is a basic and indispensable technique in microbiology. It forms the foundation for bacterial isolation, enumeration, and further identification. The technique is widely used in clinical diagnosis, environmental studies, food microbiology, and industrial microbiology.
Streak Plate Method
Introduction
The streak plate method is the most widely used technique for the isolation of bacteria in microbiology laboratories. Bacteria in natural samples usually occur as mixed populations. This method helps in separating individual bacterial cells on the surface of a solid culture medium so that they grow into well-isolated colonies, each derived from a single bacterial cell.
Definition
The streak plate method is a microbiological technique in which a bacterial sample is streaked over the surface of a solid agar medium using a sterile inoculating loop to obtain discrete and isolated bacterial colonies.
Principle
The principle of the streak plate method is mechanical dilution. During successive streaking on the agar surface, the number of bacterial cells gradually decreases. This results in the separation of individual cells, which upon incubation multiply to form isolated colonies.
Materials Required
- Nutrient agar plate
- Inoculating loop (nichrome or platinum)
- Bacterial culture/sample
- Spirit lamp/Bunsen burner
- Incubator
Procedure
- Sterilize the inoculating loop by flaming and allow it to cool.
- Dip the loop into the bacterial sample.
- Gently streak the sample over the first quadrant of the agar plate.
- Sterilize the loop again and streak from the edge of the first quadrant into the second quadrant.
- Repeat the process for the third and fourth quadrants, sterilizing the loop each time.
- Incubate the plate in an inverted position at suitable temperature (usually 37°C).
- After incubation, observe isolated colonies in the final streaks.
Types of Streaking Patterns
- Quadrant streaking
- T-streak method
- Continuous streak method
Applications
- Isolation of pure bacterial cultures
- Study of colony morphology
- Identification of bacteria
- Antibiotic sensitivity testing
- Routine microbiological analysis
Advantages
- Simple and rapid technique
- Requires minimal equipment
- Highly effective for pure culture isolation
- Economical and widely applicable
Limitations
- Not suitable for strict anaerobic bacteria
- Requires skilled handling for proper isolation
- Not useful for quantitative bacterial counts
Precautions
- Maintain strict aseptic conditions
- Sterilize the loop properly between streaks
- Avoid damaging the agar surface
- Do not open the Petri dish unnecessarily
Significance
The streak plate method forms the basis of bacterial isolation in microbiology. It is extensively used in clinical laboratories, research institutions, and teaching laboratories for obtaining pure cultures essential for further biochemical and molecular studies.
Spread Plate Method
Introduction
The spread plate method is an important microbiological technique used for the isolation and enumeration of viable bacteria. It is especially useful when the bacterial population in a sample is high and needs to be reduced by dilution. In this method, a known volume of diluted bacterial suspension is spread uniformly over the surface of a solid agar medium to obtain well-separated colonies.
Definition
The spread plate method is a technique in which a measured volume of a diluted bacterial sample is spread evenly over the surface of a solid agar medium using a sterile spreader to obtain isolated surface colonies.
Principle
The principle of the spread plate method is uniform distribution of bacterial cells over the agar surface. When the sample is sufficiently diluted, individual bacterial cells are spatially separated. Each viable cell multiplies during incubation to form a distinct surface colony, allowing both isolation and counting of bacteria.
Materials Required
- Sterile nutrient agar plates
- Diluted bacterial sample
- Sterile glass spreader (L-rod)
- Spirit lamp/Bunsen burner
- Incubator
Procedure
- Prepare serial dilutions of the bacterial sample.
- Pipette a known volume (usually 0.1 mL) of diluted sample onto the center of a sterile agar plate.
- Sterilize the glass spreader by dipping in alcohol and flaming; allow it to cool.
- Spread the sample evenly over the entire agar surface by rotating the plate.
- Allow the liquid to absorb into the agar.
- Incubate the plate in an inverted position at suitable temperature (generally 37°C).
- After incubation, observe well-separated surface colonies.
Applications
- Isolation of pure bacterial cultures
- Enumeration of viable bacteria (viable plate count)
- Microbiological analysis of water, food, and milk
- Antibiotic and disinfectant testing
- Environmental and soil microbiology studies
Advantages
- Simple and reliable method
- Allows accurate quantitative estimation of bacteria
- No heat damage to bacterial cells
- Suitable for aerobic microorganisms
Limitations
- Only surface-growing (aerobic) bacteria can be cultured
- Requires proper dilution to avoid overcrowding
- Not suitable for anaerobic or microaerophilic bacteria
Precautions
- Use appropriate dilution to obtain isolated colonies
- Ensure even spreading without damaging agar
- Maintain strict aseptic conditions
- Use sterile spreader for each plate
Significance
The spread plate method is widely used in clinical, industrial, and environmental microbiology. It plays a vital role in determining microbial load in samples and in obtaining isolated colonies necessary for identification and further study.
Pour Plate Method
Introduction
The pour plate method is a classical microbiological technique used for the isolation and enumeration of bacteria. Unlike the streak and spread plate methods, in the pour plate method the bacterial sample is mixed with molten agar before solidification. As a result, bacterial colonies develop both on the surface and within the agar medium, making this method useful for organisms with different oxygen requirements.
Definition
The pour plate method is a microbiological technique in which a measured volume of a diluted bacterial sample is mixed with molten agar medium and poured into sterile Petri plates to obtain isolated colonies inside and on the surface of agar.
Principle
The principle of the pour plate method is dilution and entrapment of bacterial cells in agar. When the molten agar solidifies, bacterial cells become fixed at different depths. Each viable cell multiplies during incubation to form a separate colony, allowing isolation and viable count.
Materials Required
- Sterile nutrient agar (molten at 45°C)
- Diluted bacterial sample
- Sterile Petri dishes
- Pipette
- Water bath
- Incubator
Procedure
- Prepare serial dilutions of the bacterial sample.
- Pipette a known volume (usually 1 mL) of diluted sample into a sterile Petri dish.
- Add 15–20 mL of molten nutrient agar (maintained at about 45°C).
- Gently rotate the plate to mix the sample evenly with the agar.
- Allow the agar to solidify at room temperature.
- Incubate the plate in an inverted position at suitable temperature (generally 37°C).
- Observe colonies formed on the surface and within the agar.
Applications
- Isolation of bacteria from mixed populations
- Enumeration of viable bacteria (standard plate count)
- Microbiological examination of milk, water, and food
- Cultivation of microaerophilic bacteria
- Research and quality control laboratories
Advantages
- Useful for counting bacteria present in low concentration
- Supports growth of surface and subsurface bacteria
- More accurate viable count compared to streak method
Limitations
- Heat of molten agar may kill heat-sensitive bacteria
- Colonies inside agar are difficult to observe and pick
- Not suitable for strict anaerobes
Precautions
- Maintain agar temperature at about 45°C
- Mix gently to avoid air bubbles
- Use aseptic techniques throughout
- Select appropriate dilution to avoid overcrowding
Significance
The pour plate method is widely used in food microbiology, dairy industry, water testing, and research laboratories. It plays a key role in determining total viable bacterial count and in isolating bacteria with varying oxygen requirements.
Enrichment Culture Technique
Introduction
In natural habitats, many bacteria of interest are present in very small numbers and are often overgrown by fast-growing, dominant microorganisms. The enrichment culture technique is designed to overcome this problem by enhancing the growth of a desired bacterium while suppressing the growth of competing microorganisms. This technique plays a crucial role in the isolation of ecologically or medically important bacteria.
Definition
The enrichment culture technique is a microbiological method in which specific growth conditions or selective media are used to favor the multiplication of a particular group of bacteria, thereby increasing their relative proportion in a mixed population.
Principle
The principle of enrichment culture is selective advantage. By modifying environmental conditions such as nutrients, pH, temperature, oxygen level, or inhibitors, the desired bacterium is allowed to grow faster than others. As a result, its population increases and becomes easier to isolate on solid media.
Types of Enrichment Culture
1. Nutritional Enrichment
- Medium contains nutrients required only by the desired bacteria
- Example: Nitrogen-free medium for nitrogen-fixing bacteria
2. Chemical Enrichment
- Addition of chemicals that inhibit unwanted organisms
- Example: Alkaline pH for Vibrio species
3. Environmental Enrichment
- Adjustment of temperature, oxygen, or salinity
- Example: High salt concentration for halophilic bacteria
Procedure
- Collect the sample containing mixed microbial population.
- Inoculate the sample into a suitable enrichment medium.
- Incubate under specific conditions favoring the target bacterium.
- Subculture the enriched sample onto solid agar medium.
- Observe and isolate the desired bacterial colonies.
Examples of Enrichment Media
- Alkaline peptone water → Vibrio cholerae
- Selenite F broth → Salmonella
- Nitrogen-free medium → Nitrogen-fixing bacteria
- High-salt medium → Halophiles
Applications
- Isolation of bacteria present in low numbers
- Medical diagnosis of pathogenic bacteria
- Environmental microbiology studies
- Agricultural microbiology (nitrogen fixers)
- Industrial microbiology research
Advantages
- Effective for isolating rare or slow-growing bacteria
- Increases success rate of isolation
- Useful when direct plating fails
Limitations
- Not absolutely selective
- Over-enrichment may suppress target organism
- Requires precise control of conditions
Precautions
- Choose appropriate enrichment medium
- Avoid prolonged incubation
- Maintain aseptic conditions
- Follow correct incubation parameters
Significance
The enrichment culture technique is indispensable in microbiology for isolating specific bacteria from complex environments. It bridges the gap between mixed populations and pure culture isolation, especially for pathogens and ecologically important microbes.